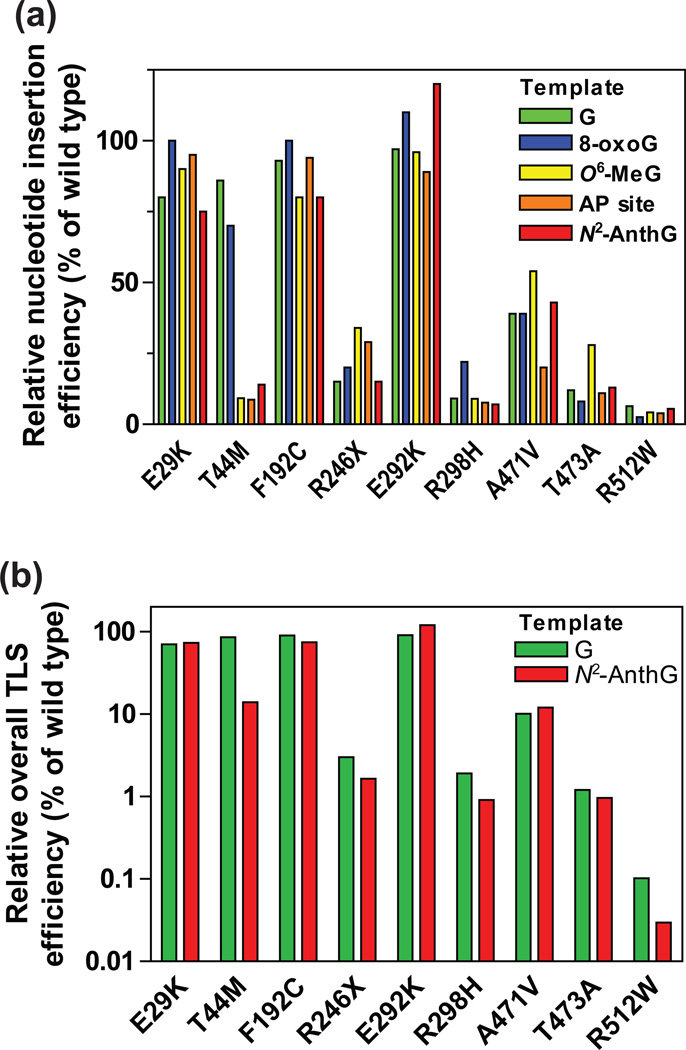
Figure 4. Relative bypass efficiencies of human variant pol κ (1–526) enzymes for G and DNA lesions compared to that of the wild-type.
(a) The relative efficiencies of nucleotide insertion opposite templates G, 8-oxoG, O6-methylG, abasic site, and N2-AnthG by variant pol κ (1–526) enzymes. The bar graph was drawn using the insertion kinetic data for insertion of the most favored dNTP opposite each template taken from the Tables 2, 3, 6, and 7. The efficiencies were normalized to that of the wild-type (set to 100%). (b) The relative efficiencies of overall TLS across (opposite and beyond) template G and N2-AnthG by variant pol κ (1–526) enzymes. The overall TLS efficiencies across G and N2-AnthG was calculated by multiplying the relative efficiency for dCTP insertion opposite G (or N2-AnthG) and the relative efficiency for the subsequent next-base extension as described previously,17 based on the insertion and extension kinetic data in Tables 2–4. The efficiencies were normalized to that of the wild-type (set to 100%).