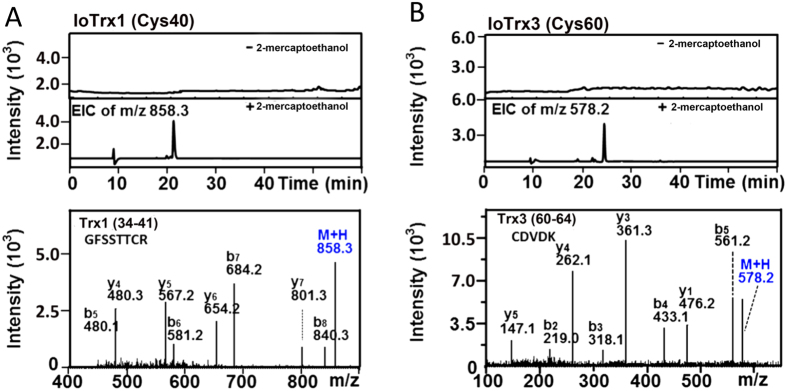
Figure 3. LC/MS/MS identification of the S-nitrosylation site in IoTrx1 and IoTrx3.
(A) SNO motif in IoTrx1; (B) SNO motif in IoTrx3. The protein-SNOs were treated by NEM for free thiol blocking and subsequently captured by thiopropyl Sepharose beads. The captured protein-SNOs were then trypsinized followed by thorough washing to remove the tryptic peptides that were not combined with the beads. The bead binding peptides (SNO-motifs) were eluted in the absence or presence of 100 mM 2-mercaptoethanol followed by LC/MS/MS analysis. In absence of 2-mercaptoethanol the flow-though did not show any extracted ion chromatography (EIC), which implies the complete blocking of free thiols (A,B, upper panels). The 2-mercaptoethanol -eluted samples presented an EIC of m/z equal to 858.3 and 578.2, which corresponded to the expected m/z values for single-charged peptide SNO peptides from IoTrx1-SNO (GFSSTTCR, residues 34–41) and IoTrx3-SNO (CDVDK, residues 60–64), respectively (A,B, lower panels).