FIG 2.
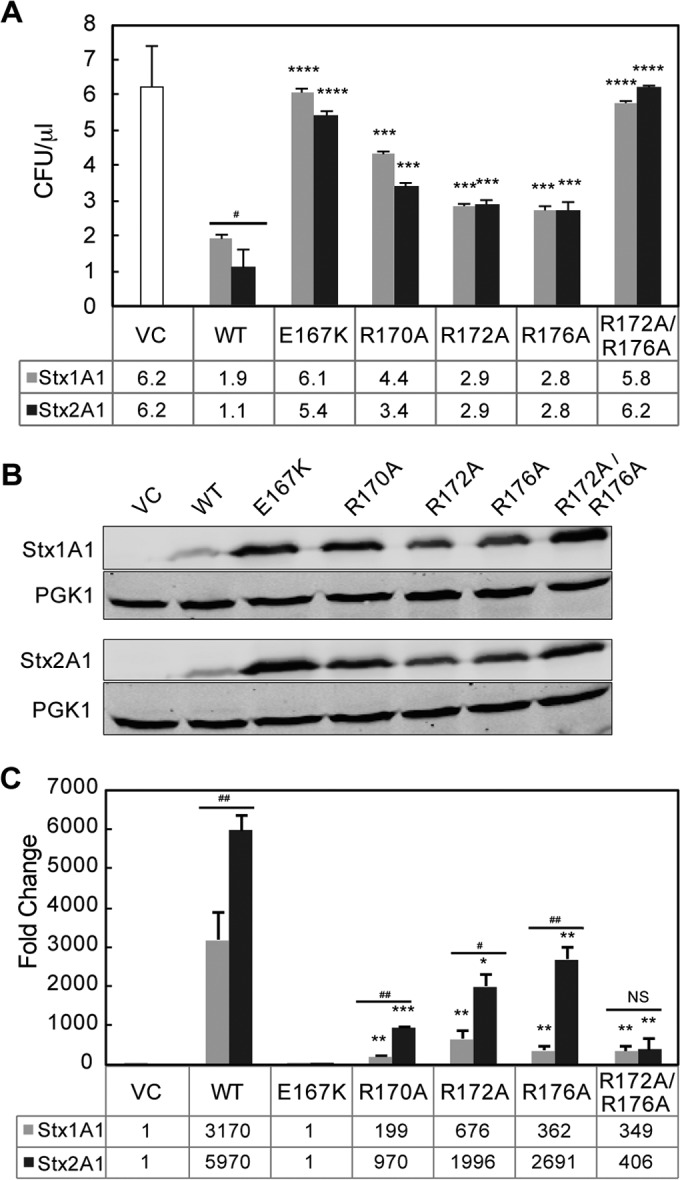
(A) Viability and ribosome depurination in yeast expressing WT or mutant Stx1A1 or Stx2A1. Yeast cells transformed with a plasmid carrying WT or mutant Stx1A1 or Stx2A1 or the empty vector (VC) were grown in SD medium supplemented with 2% glucose and then transferred to SD medium supplemented with 2% galactose. At 0 and 4 hpi, a series of 10-fold dilutions were plated on media containing 2% glucose and grown at 30°C for 1 to 2 days. The numbers of CFU per milliliter at 4 hpi were calculated from at least 3 independent transformants. The error bars represent standard errors (SE) (n = 3 independent experiments). The means of Stx1A1, Stx2A1, and their variants were significantly different using a two-sample t test (***, P < 0.001, ****, P < 0.0001 [means compared to the respective WT]; #, P < 0.05 [means compared between Stx1A1 and Stx2A1]). (B) Immunoblot analysis of yeast cells transformed with WT or mutant Stx1A1 or Stx2A1. Total protein from cells (OD600 = 5) isolated at 6 hpi was separated on an SDS-2% PAGE gel and probed with anti-V5. Anti-PGK1 was used as a loading control. (C) Depurination of ribosomes in yeast. Total RNA (375 ng) isolated from cells (OD600 = 1) expressing WT or mutant Stx1A1 or Stx2A1 collected at 1 hpi was used to quantify the relative level of depurination using qRT-PCR. The y axis shows the fold change in depurination of toxin-treated samples over the control samples without toxin (VC). The error bars represent SE (n = 3 replicates). The means of WT Stx1A1 and Stx2A1 and Stx1A1 and Stx2A1 variants were significantly different using a two-sample t test (*, P < 0.05, **, P < 0.01, ***, P < 0.001 [means compared to the respective WT]; #, P < 0.05, ##, P < 0.01 [means compared between Stx1A1 and Stx2A1]; NS, not significant).
