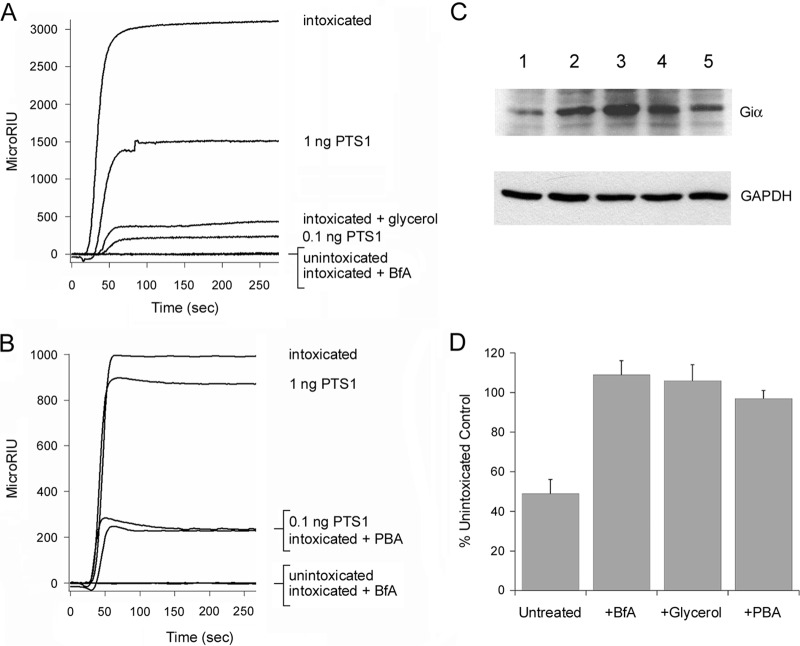
FIG 4.
Chemical chaperones inhibit PTS1 delivery to the cytosol and PT intoxication. (A and B) CHO cells pulse-loaded at 4°C with PT were chased for 3 h at 37°C in toxin-free medium containing 10% glycerol (A) or 100 μM PBA (B). Intoxicated cells chased in the absence of drug treatment were used as positive controls, while unintoxicated cells and cells intoxicated in the presence of BfA were used as negative controls. To detect the translocated toxin, cytosolic fractions from digitonin-permeabilized cells were perfused over an SPR slide coated with an anti-PTS1 antibody. PTS1 standards were perfused over the sensor as additional controls; only the 1-ng and 0.1-ng controls are shown (for scaling purposes). Ligand was removed from the perfusion buffer after 200 s. (C) CHO cells pulse-loaded at 4°C with PT were chased for 3 h at 37°C in toxin-free medium containing 10% glycerol or 100 μM PBA. Unintoxicated cells, intoxicated cells chased in the absence of drug treatment, and cells intoxicated in the presence of BfA were used as controls. Cell extracts generated by detergent lysis were incubated at 25°C for 1 h with purified PTS1 and biotin-NAD, the donor molecule for the ADP-ribosylation reaction. Since Giα can only be ADP-ribosylated by PTS1 at one site, its in vivo modification due to productive intoxication will prevent subsequent in vitro modification with biotin-labeled ADP-ribose. Western blot analysis was used to detect biotin-labeled Giα or the GAPDH loading control. Lane 1, intoxicated cells; lane 2, unintoxicated cells; lane 3, cells intoxicated in the presence of BfA; lane 4, cells intoxicated in the presence of glycerol; lane 5, cells intoxicated in the presence of PBA. (D) Signals for biotin-labeled Giα were normalized to those for the GAPDH loading control and then expressed as percentages of the values from the unintoxicated control cells. The averages ± standard deviations of results from 3 to 5 independent experiments per condition are shown.