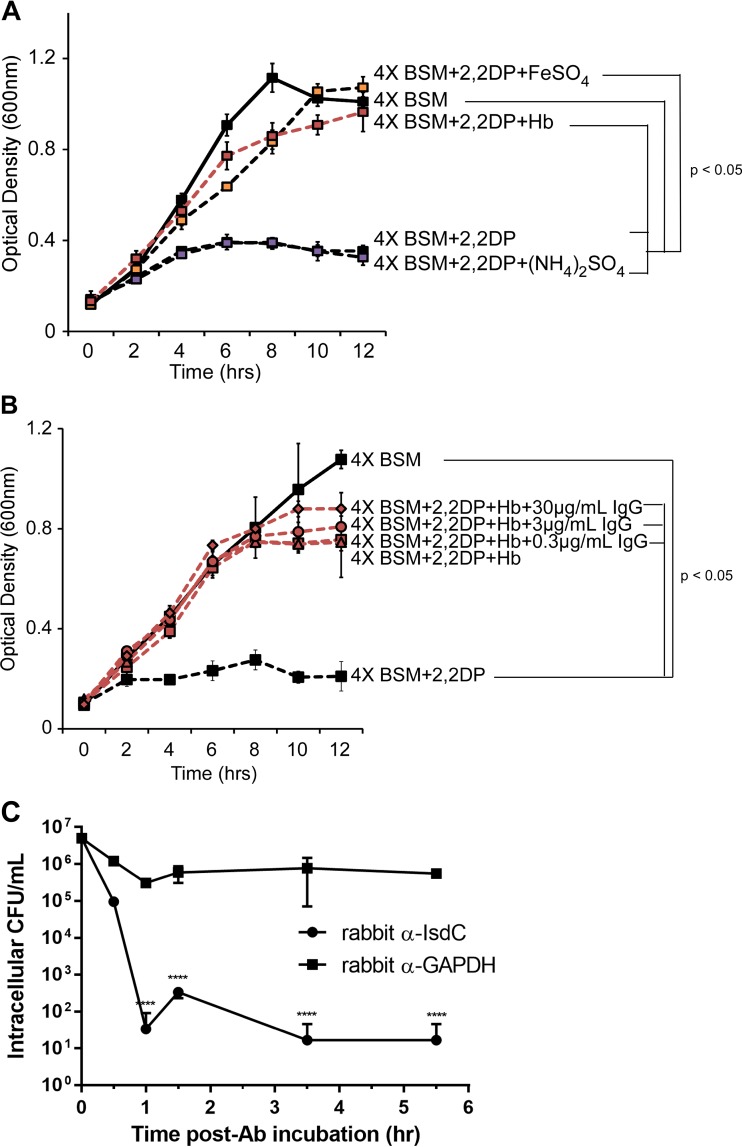
FIG 4.
Effect of anti-NEAT antibodies on iron intake or opsonin-mediated macrophage killing. (A and B) B. anthracis was cultured in 4× BSM (solid lines) or 4× BSM with 2,2-dipyridyl (2,2DP) (500 μM) (dashed lines) in the presence of iron sulfate (150 μM) (orange boxes), ammonium sulfate (150 μM) (purple boxes), and human hemoglobin (Hb) (15 μM) (red lines) (A) or hemoglobin with increasing concentrations of purified rabbit anti-IsdC (red symbols) (B), and growth was measured by absorbance spectroscopy (OD600). Each data point represents the average of results from three independent cultures ± standard deviations. (C) Purified rabbit anti-IsdC or a purified rabbit isotype control (anti-GAPDH), each at 30 μg/ml, was preincubated with B. anthracis strain Sterne for 30 min at 37°C, washed, and added to MH-S alveolar macrophages (MOI of 5), with phagocytosis being synchronized by low-speed centrifugation. At each designated time point, MH-S cells were washed with 1× PBS, incubated with gentamicin for 30 min to eliminate extracellular bacteria, and lysed with Triton X-100. Intracellular bacterial counts were determined by serial plating of the lysed samples. The values represent the means and standard deviations of data from three independent experiments. Data were analyzed by using an unpaired t test comparing the average values at each time point (****, P < 0.00001). Ab, antibody.