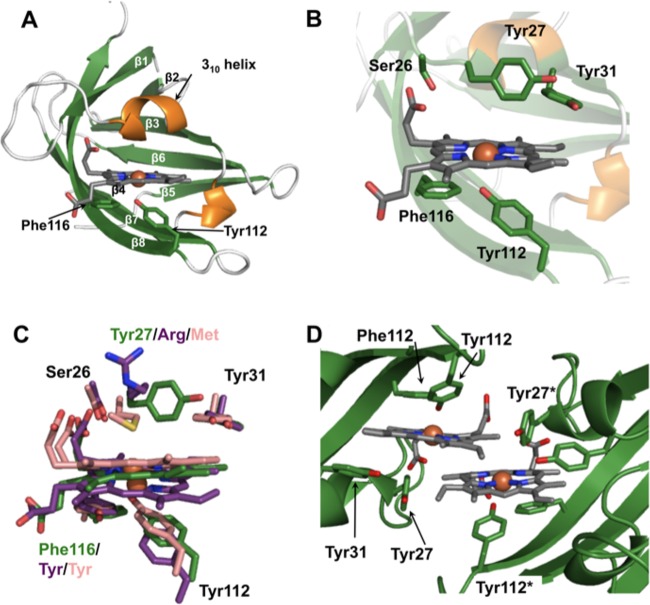
FIG 5.
Structure of heme-HalN. (A) Ribbon representation of heme-HalN, where β-strands and α-helices are shown in green and orange, respectively. Important heme-binding residues are shown in stick representation with carbon, oxygen, and nitrogen atoms in green, red, and blue, respectively, and heme is also in stick representation with carbon atoms in dark gray. (B) Closeup of the heme-binding pocket with the same color scheme as that described above for panel A. (C) Superimposition of the heme-binding sites of HalN, IsdX1, and IsdX2N5, where important heme-binding residues and heme are in stick representation, with carbon atoms of HalN in green, IsdX1 in pink, and IsdX2N5 in purple. (D) View of the dimeric interface of HalN, which is mediated predominantly by the two heme molecules. “*” denotes the second molecule in residue numbering (colored as described above for panel A).