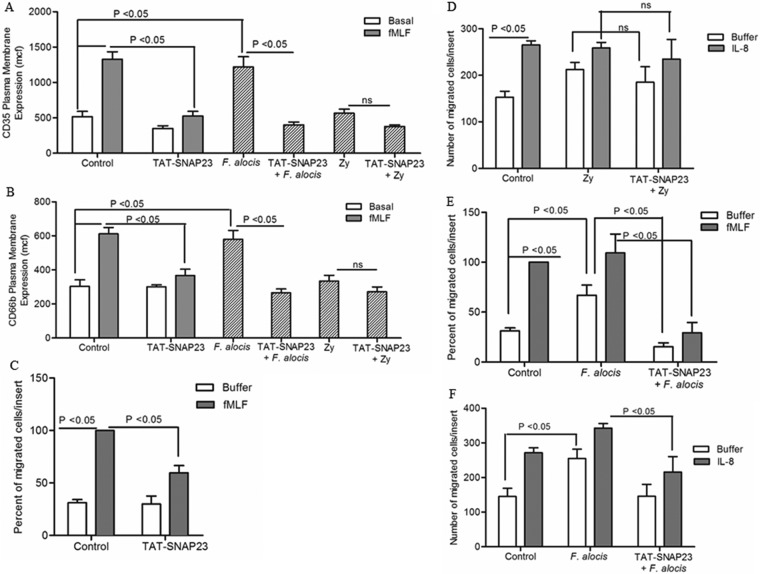
FIG 7.
Blocking neutrophil granule exocytosis inhibits F. alocis-induced random and directed migration. Neutrophils were left unchallenged (control), stimulated with fMLF (300 nM, 5 min), treated with TAT-SNAP23 (10 min), pretreated with TAT-SNAP23 followed by fMLF stimulation, challenged with F. alocis (30 min), challenged with zymosan (Zy; 30 min), pretreated with TAT-SNAP23 (10 min) followed by F. alocis challenge (TAT-SNAP23 + F. alocis), or pretreated with TAT-SNAP23 followed by zymosan challenge (TAT-SNAP23 + Zy). (A and B) Secretory vesicle and specific granule exocytosis were determined by the increase in plasma membrane expression of the CD35 or CD66b marker, respectively, by flow cytometry. Data are expressed as mean mcf ± SEM from 5 independent experiments. (C to F) Following cell stimulation or bacterial challenge, cells were placed in the upper chamber of the transwell system. After 30 min of incubation, the membrane was stained with a HEMA 3 stain set kit. Chemotaxis was assessed by light microscopic examination (magnification, ×100). (C and E) Buffer or fMLF (100 nM) was placed in the lower well. Data are means ± SEM from 5 independent experiments. (D and F) Buffer or IL-8 (100 ng/ml) was placed in the lower well. Data are expressed as mean (±SEM) number of migrated cells/insert from 5 independent experiments (D) and from 7 independent experiments (F).