FIG 1.
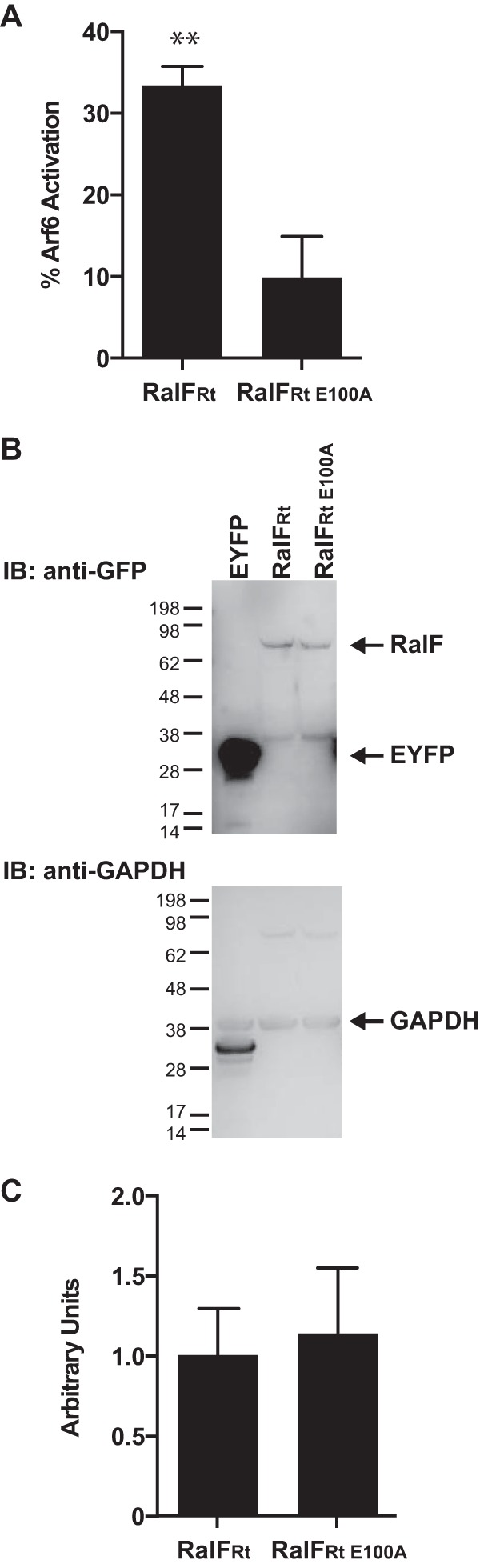
RalFRt activates Arf6. (A) HeLa cells overexpressing EYFP, EYFP-RalFRt, or EYFP-RalFRt E100A were assayed for Arf6 activation using the G-LISA Arf6 Activation Assay Biochem kit (Cytoskeleton). The percentage of Arf6 activation calculated relative to cells transfected with EYFP only is shown. Error bars represent means ± standard errors of the means (SEM) from three independent experiments. EYFP-RalFRt, but not EYFP-RalFRt E100A, significantly increased Arf6 activation compared to the level with EYFP alone. **, P < 0.01 by one-way ANOVA and Dunnett's multiple-comparison test. (B) Protein immunoblot (IB) of representative lysates assayed for Arf6 activation. HeLa cells ectopically expressing EYFP, EYFP-RalFRt, or EYFP-RalFRt E100A were lysed, and equal concentrations of cellular lysate were analyzed by protein immunoblotting using an anti-GFP antibody and reprobed with anti-GAPDH antibody as indicated. The additional bands migrating near EYFP for EYFP-RalFRt and EYFP-RalFRt E100A are potentially cleaved EYFP or a nonspecific binding product. (C) Densitometry analysis using ImageJ software (NIH) was performed to ensure equal quantities of EYFP-RalFRt and EYFP-RalFRt E100A relative to GAPDH in the lysates used for the Arf6 activation assays (A). The means ± SEM from three independent experiments are plotted. No statistically significant difference in densitometry was detected using a Student's two-sided t test (n = 3).
