FIG 2.
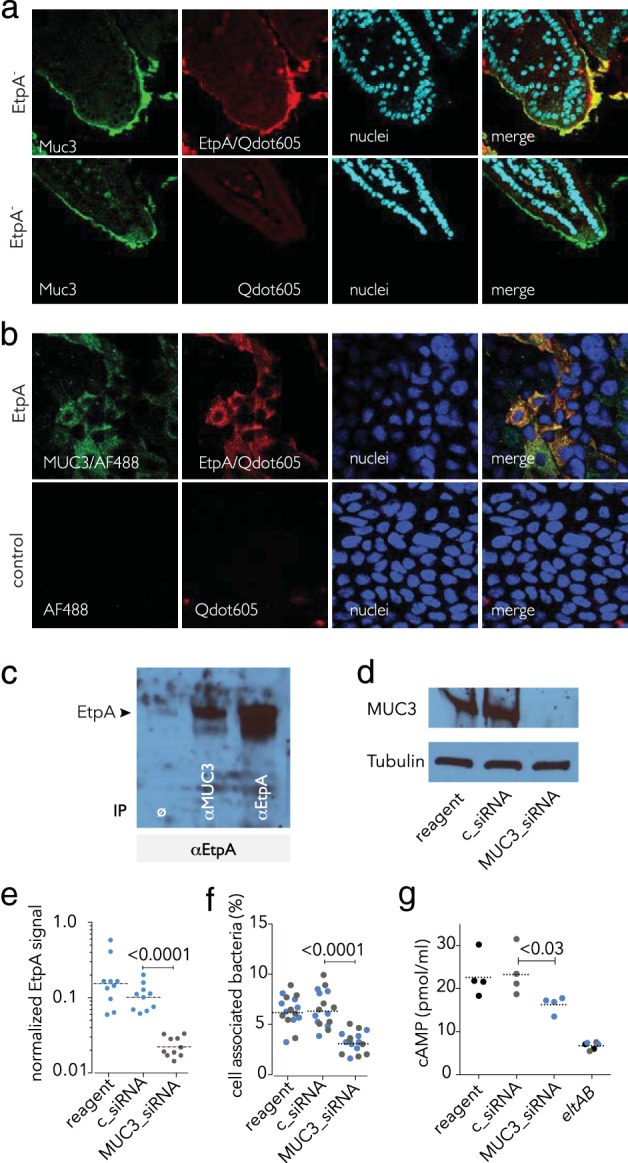
EtpA interaction with cell surface MUC3 is required for optimal ETEC epithelial cell interaction and toxin delivery in vitro. (a) EtpA interacts with Muc3 in the murine small intestine. The top row shows rEtpA (biotinylated) binding to the surface of ileal sections detected with Qdot 605. Negative controls lack rEtpA (row 2). (b) EtpA colocalizes with MUC3 on the apical surface of intestinal enterocytes. The top row shows biotinylated EtpA binding (detected with SA-Qdot 605) to Caco-2 BBe cells and colocalization with MUC3 (green); negative controls (no EtpA, no anti-MUC3 primary antibody) are shown in the bottom row. (c) EtpA immunoblot assay showing EtpA coimmunoprecipitation studies. Lanes: 1, no-antibody negative control (ø); 2, coimmunoprecipitation with anti-MUC3 antibody; 3, anti-EtpA antibody (+immunoprecipitation [IP] control). (d) siRNA-mediated depletion of MUC3 from the surface of Caco-2 epithelial cells. Shown in the immunoblot assay at the top is MUC3 produced by Caco-2 epithelial cells following treatment with transfection reagent alone (lane 1), control siRNA (lane 2), or MUC3 siRNA (lane 3). Tubulin was used as a loading control. (e) Depletion of cell surface MUC3 leads to diminished EtpA interaction with Caco-2 cells. Data represent EtpA immunofluorescence signals quantitated with Volocity imaging analysis software in MUC3 siRNA-treated cells and controls. (f) Optimal ETEC interaction with epithelial cells requires MUC3. Shown are bacteria adherent to MUC3 siRNA-treated cells and controls. (Colors represent results from two independent experiments performed on different days). (g) Optimal delivery of LT by ETEC requires MUC3. The LT-negative (eltAB) mutant was included as a negative control. Dashed horizontal lines represent geometric mean values. Statistical calculations were performed by Mann-Whitney two-tailed nonparametric testing.
