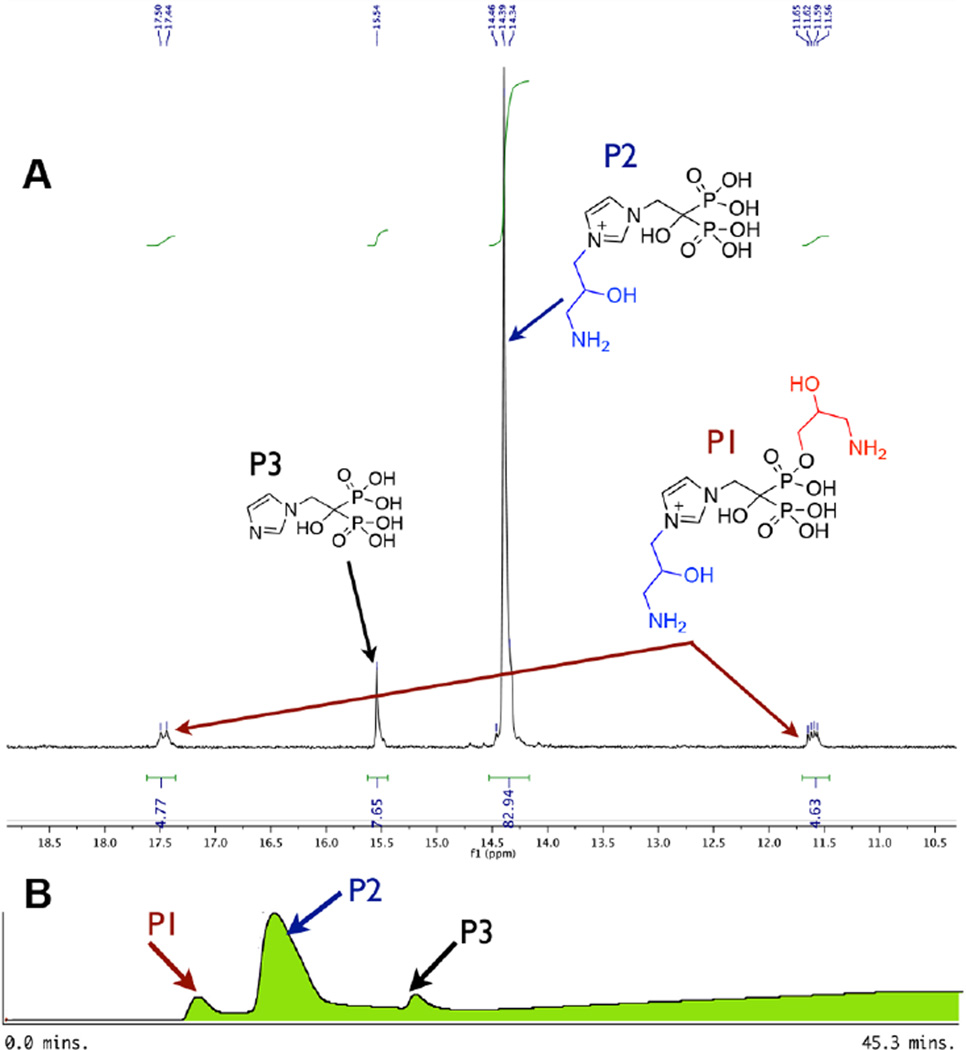
Figure 1.
Reaction of ZOL (1d) and 5. (A) 31P NMR of reaction mixture of 1d and 5 via Route A (in D2O, pH 7.5; 50 °C for 41 h then deprotection with TFA:H2O (1:1)). (B) HPLC analysis of reaction mixture of ZOL (1d) and 5 using a strong anion exchange (SAX) column (Macherey-Nagel 21.4 mm × 150 mm SP15/25 Nucleogel column), eluted with eluent a, H2O; b, 0.5 M TEAB pH 7.5, gradient increased from 0% to 30% over 10 min, then maintained at 30% during 10–15 min, then increased to 100% of eluent b from 15 to 35 min at 9 mL/min flow rate; UV–vis detection at 230 nm.