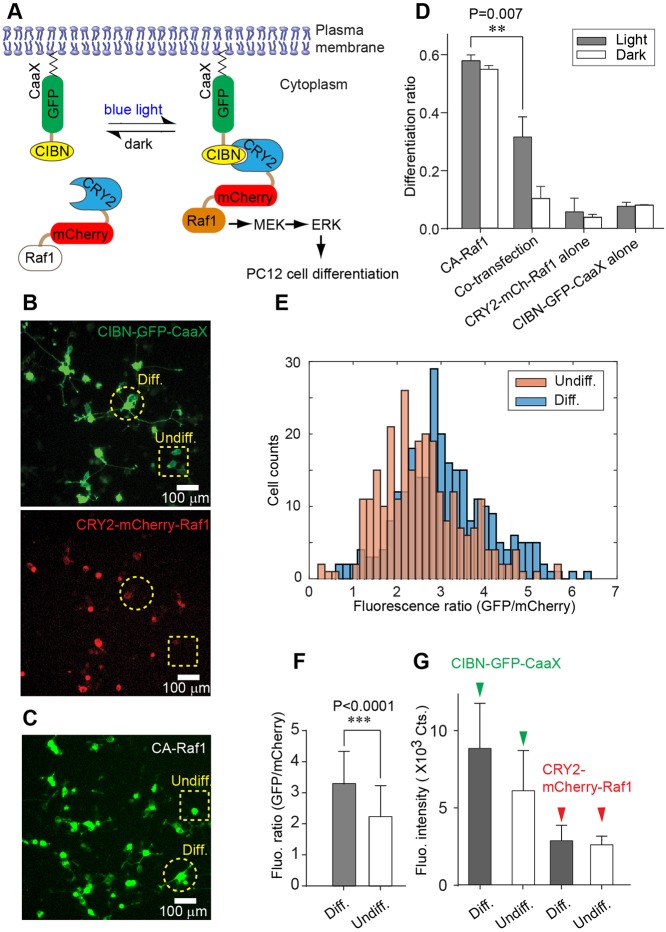
Fig. 1.
Correlation between the differentiation ratio and the fluorescence intensity ratio (GFP/mCherry) in PC12 cells co-transfected with CIBN-GFP-CaaX and CRY2-mCherry-Raf1. (A) Light-induced binding between CIBN and CRY2 leads to membrane recruitment of CRY2-mCherry-Raf1, which activates the Raf/MEK/ERK signaling pathway and induces PC12 cell differentiation. (B) Representative fluorescence images of PC12 cells co-transfected with CIBN-GFP-CaaX (green) and CRY2-mCherry-Raf1 (red). A differentiated cell is marked with a dashed circle; an undifferentiated cell is marked with a dashed square. Differentiated cells are defined as those with at least one neurite of length equal to or longer than the diameter of their cell bodies. (C) A representative image of cells transfected with Raf1-GFP-CaaX, a constitutively active Raf1 (CA-Raf1). A differentiated cell is marked with a dashed circle; an undifferentiated cell is marked with a dashed square. (D) Differentiation ratios of PC12 cells transfected with CA-Raf1, co-transfected with CRY2-mCherry-Raf1 and CIBN-GFP-CaaX, singly transfected with CRY2-mCherry-Raf1, or singly transfected with CIBN-GFP-CaaX. Twenty four hours after transfection, cells were either exposed to light (0.2 mW/cm2) or incubated in the dark for another 24 h. Values represent mean±s.d. from four independent data sets. (E) Histograms of fluorescence intensity ratio (GFP/mCherry) for differentiated and undifferentiated cells in co-transfected cultures. Number of cells analyzed: differentiated cells (n=430), undifferentiated cells (n=430). (F) Quantification of GFP/mCherry ratio for the data shown in E. Values represent mean±s.d. Differentiated cells show a significantly higher GFP/mCherry ratio than undifferentiated cells. P-value was determined by a two-tailed, unpaired t-test. (G) Fluorescence intensities of GFP and mCherry in differentiated and undifferentiated cells. Values represent mean±s.d. P-values (D,F) were determined by a two-tailed, unpaired t-test.