ABSTRACT
Animals need to flexibly respond to stimuli from their environment without compromising behavioural consistency. For example, female crickets orienting toward a conspecific male's calling song in search of a mating partner need to stay responsive to other signals that provide information about obstacles and predators. Here, we investigate how spontaneously walking crickets and crickets engaging in acoustically guided goal-directed navigation, i.e. phonotaxis, respond to mechanosensory stimuli detected by their long antennae. We monitored walking behaviour of female crickets on a trackball during lateral antennal stimulation, which was achieved by moving a wire mesh transiently into reach of one antenna. During antennal stimulation alone, females reduced their walking speed, oriented toward the object and actively explored it with antennal movements. Additionally, some crickets initially turned away from the approaching object. Females responded in a similar way when the antennal stimulus was presented during ongoing phonotaxis: forward velocity was reduced and phonotactic steering was suppressed while the females turned toward and explored the object. Further, rapid steering bouts to individual chirps, typical for female phonotaxis, no longer occurred. Our data reveal that in this experimental situation, antennal stimulation overrides phonotaxis for extended time periods. Phonotaxis in natural environments, which require the integration of multiple sensory cues, may therefore be more variable than phonotaxis measured under ideal laboratory conditions. Combining this new behavioural paradigm with neurophysiological methods will show where the sensory-motor integration of antennal and acoustic stimulation occurs and how this is achieved on a mechanistic level.
KEY WORDS: Phonotaxis, Antenna, Tactile sensing, Bimodal sensory integration, Trackball
Summary: Investigation of the effect of antennal mechanosensory stimulation in female crickets walking on a trackball shows that phonotaxis to male calling song is suppressed while the animals explore objects with their antennae.
INTRODUCTION
Mating success reflects an animal's fitness (Rodríguez-Muñoz et al., 2010; Simmons, 1988). This renders mating behaviour a convenient system for behavioural studies as it combines behavioural robustness and low variability with a range of interesting computational problems such as multi-modal sensory integration, the control of complex behavioural sequences and action selection. We present a novel behavioural paradigm that allows studying bimodal integration in the context of cricket mating behaviour.
The mating behaviour of female field crickets consists of several stages: acoustically guided approach of a mate, close-range courtship and, finally, copulation (Adamo and Hoy, 1994). We focus on the first stage, during which female crickets orient and walk toward a male's calling song while searching for a mating partner, a well-studied behaviour known as phonotaxis (Adamo and Hoy, 1994; Murphey and Zaretsky, 1972; Regen, 1913).
Most studies of phonotaxis have been performed under tightly controlled laboratory conditions as a purely auditory orientation task (Gerhardt and Huber, 2002; Schmitz et al., 1982; Weber and Thorson, 1989). However, in its natural habitat, a female cricket has to navigate through potentially dense grassland while tracking the male's calling song. Under these conditions its phonotactic behaviour will be affected by other environmental stimuli, which could influence course control. Indeed, tracking speed and tracking accuracy of female crickets performing phonotaxis are lower when measured in the field compared with results obtained under controlled laboratory conditions (Hirtenlehner and Römer, 2014; Hirtenlehner et al., 2014).
Besides auditory cues, mechanosensory stimuli perceived via the antennae may play an important role in course control. Antennal detection and exploration of objects during navigation has been described in cockroaches (Harley et al., 2009; Okada and Toh, 2006; Ritzmann et al., 2012). Walking crickets also constantly move their long antennae, sampling the near space ahead (Horseman et al., 1997).
We characterize responses to mechanosensory antennal stimulation in walking crickets and investigate how they affect phonotaxis and whether they are modulated during phonotaxis. To do this, we have developed a behavioural paradigm in tethered crickets that combines a well-established experimental approach for studying steering manoeuvres during phonotaxis (Hedwig and Poulet, 2005) with antennal mechanosensory stimulation. While previous studies of antennal sensing in walking insects have used solid objects such as a plate or a rod (Okada and Toh, 2006; Ritzmann et al., 2012; Schütz and Dürr, 2011), we chose a sound-transparent metal wire mesh, which allowed us to deliver antennal and acoustic stimulation independently. We stimulated the antennae by moving the wire mesh into antennal reach of tethered female crickets walking in darkness on a trackball. To investigate how potentially conflicting steering manoeuvres are integrated during phonotaxis, we measured responses to antennal stimulation both in the absence and in the presence of acoustic stimulation.
MATERIALS AND METHODS
Animals
All experiments were performed with virgin female crickets (Gryllus bimaculatus DeGeer 1773). Animals were isolated before their final moult, and tested at least 10 days after, to ensure phonotactic responsiveness. At least 1 day before the first behavioural experiments, an insect pin, which served as a tether, was waxed onto the first abdominal tergite.
Experimental setup
Females were positioned in normal walking posture and tethered above a freely rotating trackball. The trackball system (details in Hedwig and Poulet, 2005) was used to record each cricket's forward walking and steering velocity. The trackball system was placed in a chamber lined with sound-damping foam, which was closed during experiments to exclude environmental light and noise. Two speakers were installed at a distance of 57 cm from the trackball at 45 deg off the longitudinal axis of the cricket, to provide acoustic stimuli from the left and right sides (Fig. 1A). An acoustically transparent metal mesh (60×65 mm, 0.7 mm wire thickness, 5.0×3.0 mm openings) was used to generate a mechanosensory stimulus. It was attached to a linear motor (LM1247-060-01 Quickshaft Linear Motor, Faulhaber, Schoeneich, Germany) allowing controlled movement toward and away from the cricket's right antenna along the radial axis at 45 deg (Fig. 1A). Video recordings (Common Vision Blox, Stemmer Imaging, Puchheim, Germany) were conducted at 60 Hz frame rate with a camera (DALSA Genie-HM640, Stemmer Imaging, Surrey, UK) placed above the cricket under illumination with red light (690 nm, LED, ELJ-690-629, Roithner Lasertechnik, Vienna, Austria), to which cricket eyes are insensitive (Labhart et al., 1984).
Fig. 1.
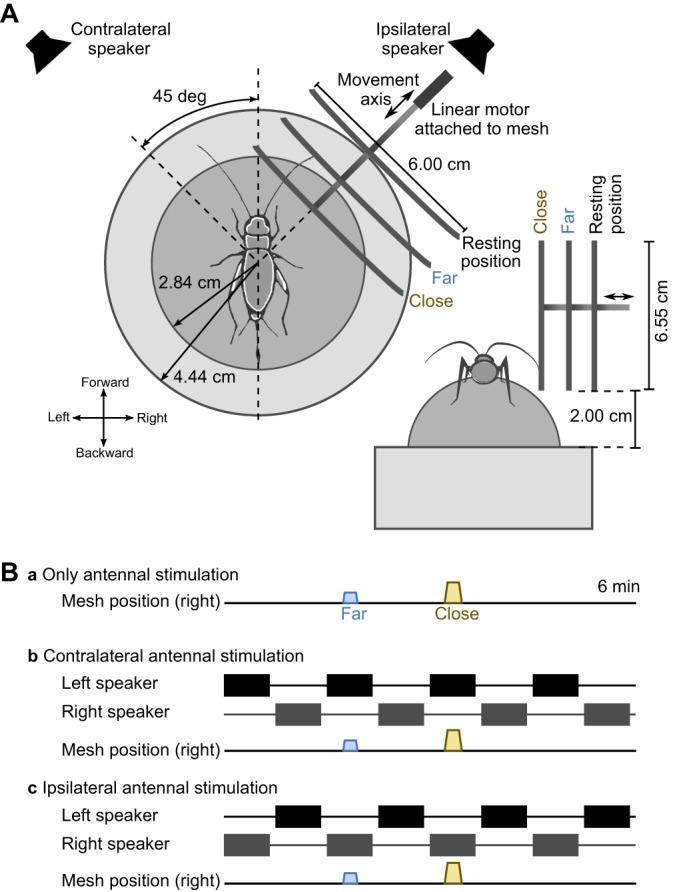
Setup and experimental protocol. (A) Arrangement of the antennal stimulation setup. The cricket is placed on a trackball. A metal mesh can be moved into and out of reach of the cricket's right antenna. Two loudspeakers are placed 57 cm away from the animal at 45 deg to the left and right. (B) Building blocks of the experimental protocols. Three protocols for acoustic stimulation are combined with two presentation orders of the antennal stimulus, resulting in a total of six test conditions.
Behavioural paradigm
To elicit phonotactic walking, the calling song of G. bimaculatus was played alternatingly from the left and the right speaker in 40-s blocks. The parameters of the artificial calling song were chosen based on previous studies to elicit maximal phonotactic steering (Hedwig and Poulet, 2005).
To achieve antennal stimulation, the metal mesh was presented at a ‘far’ position, where the mesh could only be touched by the very tip (2–5 mm) of the antenna, or at a ‘close’ position, which was 1 cm nearer. Because body size and antennal length vary across animals, we determined for each animal individually the far and close distance. Presentation of the metal mesh will be referred to as far and close antennal stimulation, respectively (Fig. 1A). Whenever no antennal stimulation was intended, the object was moved into a resting position out of antennal reach. The movement of the mesh between the resting position and the presentation position took 1.25 and 2.5 s for the far and close positions, respectively. The beginning of the antennal stimulation period is referred to as time 0; it is defined as the moment at which the object reached its trial-specific, i.e. far or close, position.
The cricket's response to 10 s long presentation of the object was tested under three stimulus configurations: (1) antennal stimulus presented alone, (2) antennal stimulus presented simultaneously with acoustic stimulation from the same side, i.e. ipsilateral, or (3) antennal stimulus presented from the opposite side, i.e. contralateral (Fig. 1B). Within one trial the object was presented once at the far and once at the close position. Each experimental condition was tested twice, presenting the mesh at the two distances in both possible orders. This resulted in a total of six trials per animal, which were tested in random order.
Responses to 30 s long antennal stimulation were not measured in spontaneously walking crickets, but only in crickets engaging in phonotaxis, and only one trial per animal was recorded measuring first responses to far and then to close antennal stimulation.
Analysis of walking velocities
The data were stored on file using LabView software (National Instruments 5.01) and subsequently processed with MATLAB (MathWorks, Natick, MA, USA). Trials were excluded from analysis if a cricket did not walk for most of the trial or did not show clear phonotactic steering. This selection procedure led to different sample sizes among experimental groups ranging from 14 to 19 out of the 31 tested animals (see Table S1 for details).
All statistical analysis was performed using R (version 3.0.0) on data that were binned into 5 s time intervals. In trials with 10 s long antennal stimulation, we focused the statistical analysis on three time intervals, ‘pre’, ‘stim’ and ‘post’, chosen to cover the time before, during and directly after antennal stimulation, respectively. For the ‘pre’ interval we chose seconds [−10, −5), i.e. all data points between −10 s and −5 s, including −10 s but excluding −5 s. The first half of the antennal stimulation period with seconds [0, 5) was chosen for the ‘stim’ interval, and seconds [10, 15) for the ‘post’ interval. Thus, the ‘post’ interval covers a time period during which crickets might still interact with the retracting mesh. In trials with 30 s of antennal stimulation we performed statistical tests on a ‘pre’ time interval and a ‘stim’ interval defined as above. In an ANOVA we further compared all six 5 s intervals covering the full antennal stimulation period. The placement of the time intervals is also indicated above the time axis in Figs 2C, 3B, 4B and 6B.
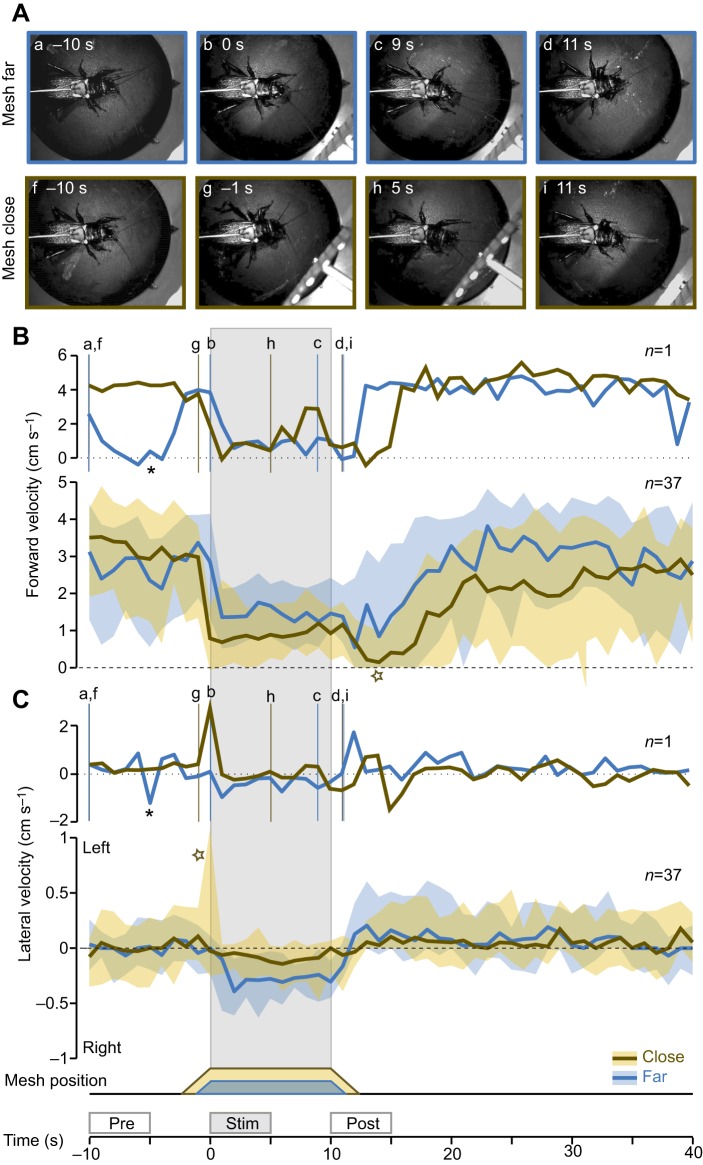
Fig. 2.
Responses to antennal stimulation in spontaneously walking crickets. (A) Video stills recorded during a ‘far’ (top, blue frame) and a ‘close’ (bottom, ochre frame) antennal stimulation trial. The corresponding forward and lateral walking velocities, which were recorded from the trackball, are shown in the upper panel of B and C. Positive and negative forward velocities indicate forward and backward movement, respectively. Lateral walking velocities of 0 cm s−1 indicate straight walking; positive and negative lateral velocities indicate steering toward the left and right side, respectively. (B) Time course of the forward velocity over a 50 s time window averaged over 1 s time intervals. Responses to far (blue line) and close (ochre line) antennal stimulation are superimposed. We show a 10 s interval before the object presentation as a reference, then 10 s of antennal stimulation (highlighted by grey shading) beginning at time 0, followed by 30 s succeeding the antennal stimulation. The upper panel shows a single trial and the lower panel the median and IQR of n=37 trials from 20 animals with one to two trials per animal. Vertical lines mark the time points of the video stills shown in A. (C) The time course of the lateral steering velocity over the same 50 s time window as the forward velocity. The plot layout is analogous to B. The 5 s time intervals used in the statistical analysis (‘pre’, ‘stim’ and ‘post’) are marked above the time axis. The asterisks in B and C mark a short right turn. The star in B marks an after-effect of antennal stimulation.
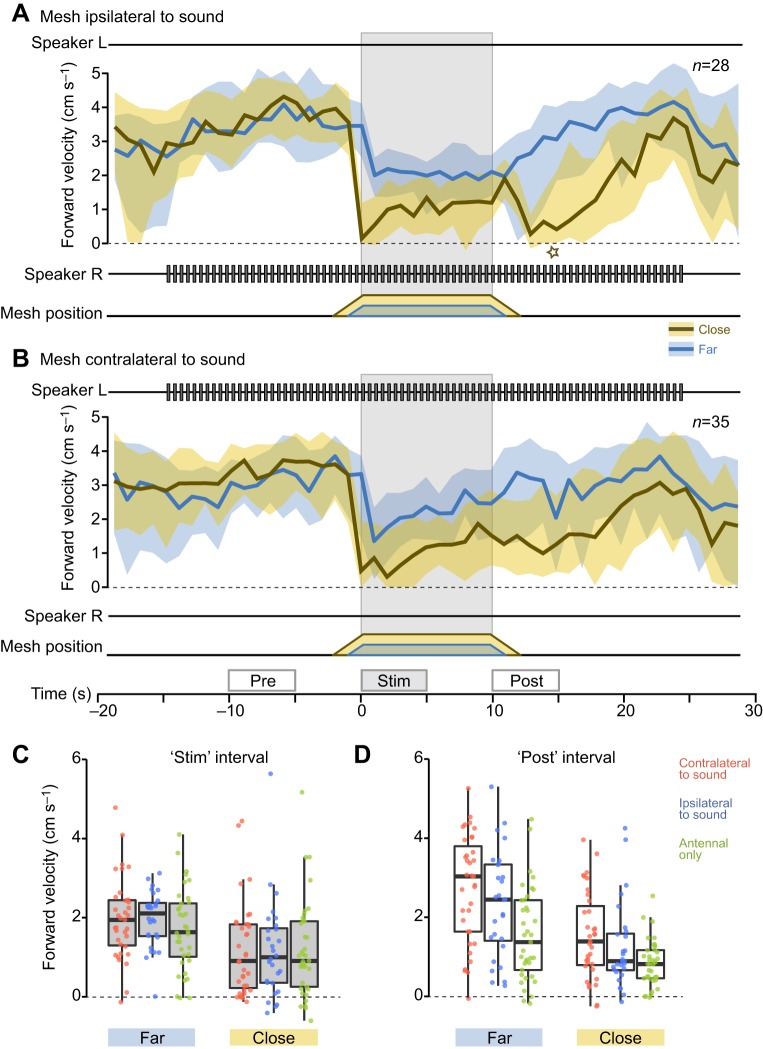
Fig. 3.
Deceleration response to antennal stimulation. (A,B) Temporal dynamics of the median forward walking velocity during a 50 s time window centred around a 40 s presentation of a male's calling song with antennal stimulation from either the right/ipsilateral side (A) or the left/contralateral side (B). The acoustic stimulus is represented by the speaker traces above and below each plot. Antennal stimulation, indicated by the mesh position trace and grey shading, occurred during seconds 0 to 10. Responses to close and far antennal stimulation trials are color-coded in ochre and blue, respectively. (A) Median and IQR of forward velocity calculated from 28 ipsilateral antennal stimulation trials (15 animals). The star marks an after-effect of antennal stimulation. (B) Median and IQR of forward velocity calculated from 35 contralateral antennal stimulation trials (20 animals). The boxes on the time axis in B mark the ‘pre’, ‘stim’ and ‘post’ intervals used in the statistical analysis. In ipsilateral trials, crickets reduced their forward speed by 3.09 cm s−1 in close and 1.34 cm s−1 in far trials, while in contralateral trials they slowed down by 2.51 cm s−1 in close and 1.08 cm s−1 in far trials (comparing ‘pre’ and ‘stim’ intervals). (C,D) Boxplots of forward velocity during the ‘stim’ (C) and ‘post’ (D) intervals. Grey fill marks data from the antennal stimulation period. For each period, data from far antennal stimulation trials are shown on the left, and data from close trials on the right. The average velocities of individual trials are overlaid on the corresponding boxplot to visualise the spread of the individual data points. Data from contralateral antennal stimulation trials are plotted in red (n=35, 20 animals), ipsilateral antennal stimulation trials in blue (n=28, 15 animals) and trials without acoustic stimulation in green (n=37, 20 animals).
Fig. 4.
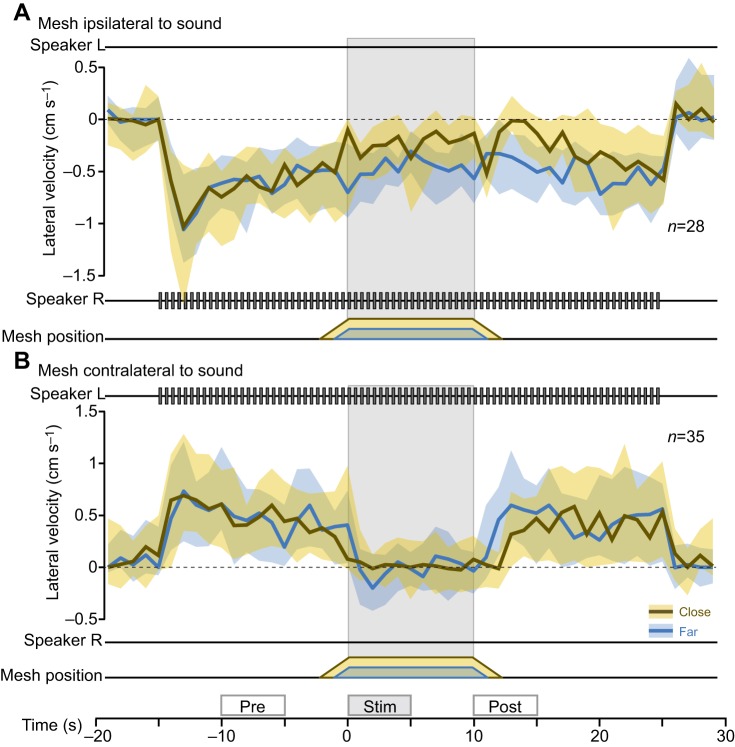
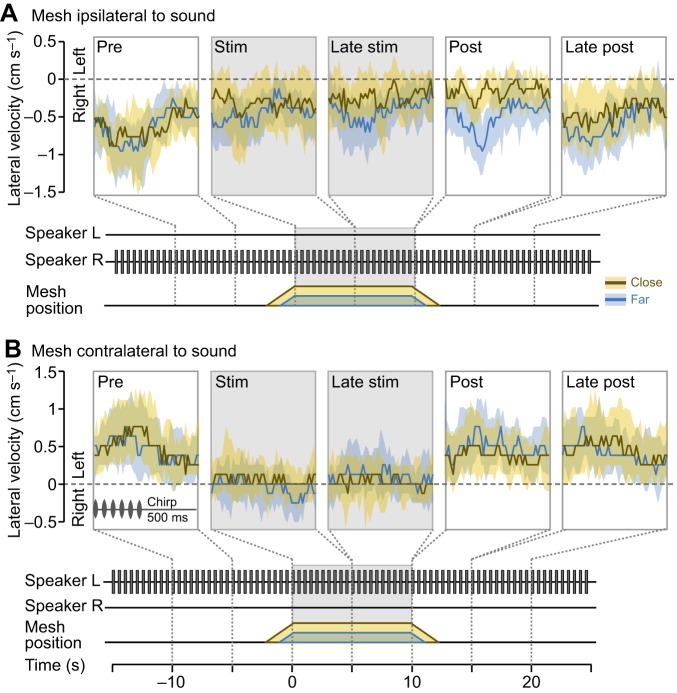
Turning responses to antennal stimulation during phonotaxis. Median lateral steering velocity during phonotaxis combined with antennal stimulation. The data are based on the same set of trials as in Fig. 3 and are presented analogously. The median and IQR of 35 ipsilateral trials from 15 animals (A) and 28 contralateral trials from 20 animals (B) are shown. The boxes above the time axis in B mark the ‘pre’, ‘stim’ and ‘post’ intervals used in the statistical analysis.
Fig. 6.
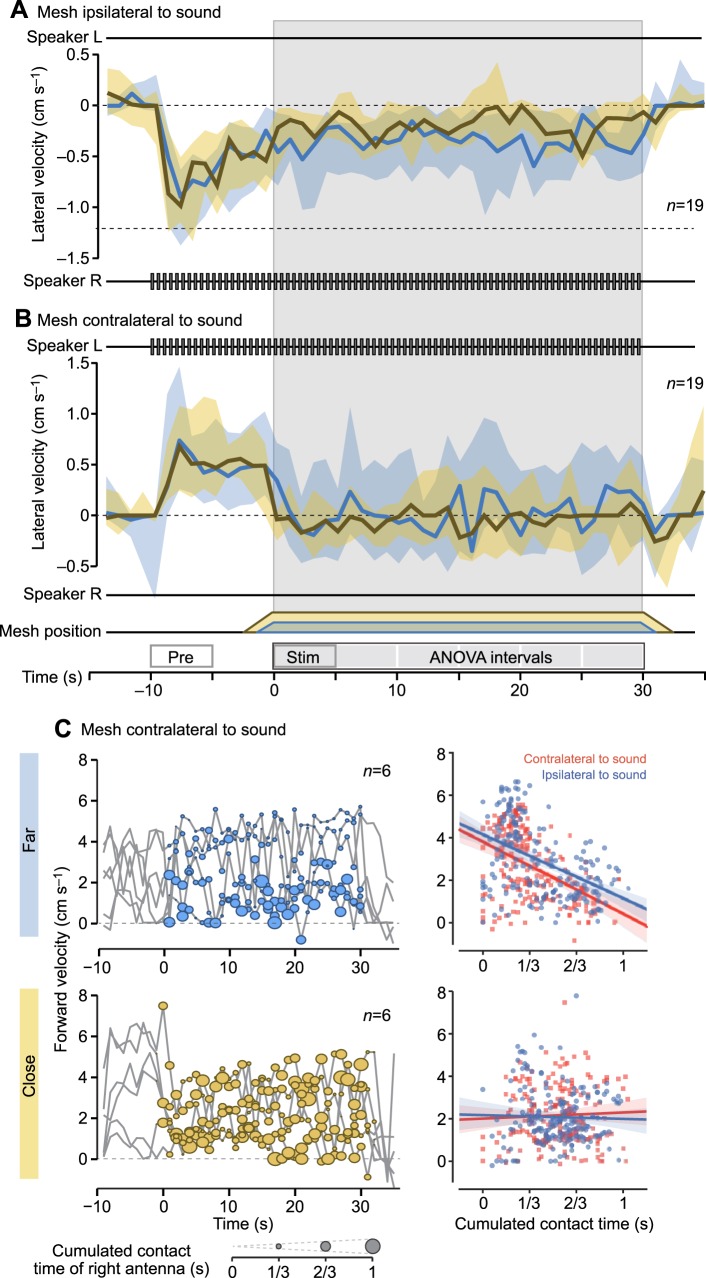
Impairment of phonotaxis during long-lasting antennal stimulation. (A,B) Temporal dynamics of the lateral velocity during phonotaxis combined with a 30 s antennal stimulation period. Layout is analogous to Fig. 4. Median and IQR of lateral steering velocity calculated from 19 ipsilateral trials (A) and 19 contralateral trials (B) are shown. In both experimental groups, the same 19 animals were tested, each contributing one trial. The boxes on the time axis in B mark the ‘pre’ and ‘stim’ intervals used in the statistical comparisons as well as the intervals that were compared in the ANOVA. (C) Antennal contacts extracted from video data overlaid onto the corresponding forward velocity trace of six crickets during contralateral antennal stimulation (seconds 0 to 30). The forward velocity and the cumulated antennal contact time were computed over 1 s time intervals. Data from far and close trials are shown in the top and bottom plots, respectively. Cumulated antennal contact time is visualised as circles, the size of which encodes the fraction of each second during which the respective cricket held antennal contact with the mesh. (D) Correlations between the forward velocity and the cumulated antennal contact time. The Pearson correlation coefficients are −0.44 (P<0.001) for far contralateral and −0.46 (P<0.001) for far ipsilateral stimulation. For close contralateral and ipsilateral stimulation, the correlation coefficients are 0.05 and −0.03, respectively (both n.s.).
Generally, the data were not normally distributed and are therefore described using median and interquartile range (IQR). For comparisons we used Wilcoxon signed rank tests for matched samples. The exact conditions, such as whether a one- or two-sided test was performed, are listed in the Results section. We also used two-way ANOVA to compare the effect of several explanatory variables on multiple groups at once.
We used two-way ANOVA to test for an effect of the order of 10 s presentation at the two mesh distances on the response during the ‘stim’ interval, but found no significant effect on lateral velocity (F1,166=2.864, P=0.095) or forward velocity (F1,166=0.154, P=0.695). Therefore, data were pooled over the presentation order for subsequent analysis.
Video analysis of antennal contact
Video frames and trackball recordings were temporally aligned using a TTL frame indicator pulse, generated by the camera. Video frames during which an antennal contact occurred were identified manually using a custom-written Python (2.7) script. Subsequently, the data were imported into Microsoft Excel and MATLAB for further processing.
RESULTS
Antennal stimulation evokes steering responses in spontaneously walking crickets
A comprehensive description of the walking and steering responses to antennal stimulation in walking crickets is required to understand how these responses are integrated with phonotaxis.
Walking crickets scan the space ahead by swinging their 2.0–2.75 cm long antennae in circulating movements. We simulated an obstacle in the cricket's walking path by positioning a metal mesh within reach of the right antenna at either a far or a close position. The cricket itself generated the mechanosensory stimulus during active exploration of the mesh. Because the mesh approached the animal in a linear movement, it came within antennal reach before it reached its final position. Video recordings showed that once the mesh came within antennal reach, the cricket repeatedly made contact, gliding over the mesh's surface with the tip of its right antenna. Upon initial antennal contact, crickets typically turned their head toward the detected object and explored it with one or both antennae (Fig. 2Ab,c,h, Movie 1).
To quantify the response to antennal stimulation, we measured the cricket's forward walking (Fig. 2B) and lateral steering velocity (Fig. 2C). For both velocity measurements we present single trials (top) and the median over all measurements (bottom). Walking velocities are illustrated over a 50 s period covering the 10 s of antennal stimulation.
The walking velocities of spontaneously walking crickets showed transient fluctuations (Fig. 2B top, C top). The low-amplitude forward velocity and the simultaneously occurring short right turn before far antennal stimulation (see asterisks in Fig. 2B,C) are examples of these animal-specific fluctuations. Averaging across trials and individuals, revealed systematic walking velocity changes in response to antennal stimulation (Fig. 2B,C, bottom).
Preceding antennal stimulation, the median forward velocity was 2.89 cm s−1 with an IQR of 2.44 cm s−1 (close and far trials pooled, ‘pre’ interval). In response to antennal stimulation we observed an abrupt reduction in forward speed. The deceleration occurred within the first second of far antennal stimulation, whereas crickets slowed down already before the start of close antennal stimulation, likely because of early antennal contacts with the approaching mesh. With a median walking speed of 0.91 cm s−1 during close and 1.68 cm s−1 during far antennal stimulation, crickets slowed down more in close trials (P<0.05, paired two-sided test, ‘stim’ interval). The cricket's forward velocity remained low throughout the antennal stimulation period independent of presentation distance. In close trials, crickets reduced their walking speed further after the mesh moved out of reach (see ochre star in Fig. 2B, bottom).
The median steering velocity of spontaneously walking crickets was close to zero (i.e. −0.05 cm s−1), indicating that there was no systematic turning bias before the antennal stimulation (IQR was 0.43 cm s−1, centred on the median; ‘pre’ interval in Fig. 2C, bottom). Within 2 s of antennal stimulation, crickets on average turned toward the mesh. Steering towards the mesh was more pronounced during far presentation as compared with close presentation; steering velocity changed significantly in far but not in close trials (far: P<0.001, close: P=0.39, paired two-sided test comparing ‘pre’ and ‘stim’ interval). Crickets kept turning toward the static mesh with a constant median velocity, i.e. the response was plateau-shaped for the duration of the stimulus presentation. After far antennal stimulation, turning toward the mesh abated within 2 s and was followed by a weak turn toward the contralateral side during the ‘post’ interval (Fig. 2C, bottom).
In some antennal stimulation trials (e.g. in the close trial presented in Fig. 2C, top), crickets initially made a fast turn away from the approaching mesh before orienting towards the stationary mesh (compare Fig. 2Ag and 2Ah, Movie 2). We observed those sharp initial turns away from the mesh in six close trials and one far trial (Fig. S1). In the median response this observation manifested itself as a peak in the IQR of the steering velocity but not in the median (see ochre star in Fig. 2C).
These data demonstrate that in walking female crickets, tactile antennal stimulation evoked a robust deceleration and a turning response directed toward the mesh in 68% of the close and 97% of the far trials. At the beginning of antennal stimulation, we observed initial rapid turns away from the approaching mesh in 16% of close trials and in 3% of far trials (Fig. S1).
Next, we investigated whether female crickets performing phonotaxis are responsive to antennal stimulation and, if so, how antennal-evoked and auditory-evoked responses presented from the same or different sides are integrated at the level of behaviour.
Crickets respond to antennal stimulation during phonotaxis with a pronounced reduction of walking speed
Phonotactic steering can be elicited in female crickets walking on a trackball by playing an acoustic model of a conspecific male's calling song (Hedwig and Poulet, 2005). We paired 10 s of antennal stimulation with an ongoing presentation of the calling song. The antennal stimulus was always presented from the right side, while the acoustic stimulus was presented from either the right, corresponding to ipsilateral stimulation (Fig. 3A), or the left, resulting in contralateral stimulation (Fig. 3B).
Crickets showed generally higher forward velocity and lower variance during phonotaxis compared with spontaneous walking: the median forward velocity was 4.05 cm s−1 in ipsilateral trials and 3.24 cm s−1 in contralateral trials (Fig. 3A,B, ‘pre’ interval, close and far trials pooled), which was considerably faster than the median spontaneous walking velocity, 2.89 cm s−1 (see Fig. 2B).
Like spontaneously walking crickets, animals engaging in phonotaxis abruptly slowed down at the onset of antennal stimulation (Fig. 3A,B) and significantly reduced their forward speed compared with pre-stimulation levels in ipsilateral as well as contralateral trials (both: P<0.001 in ANOVA over ‘pre’ and ‘stim’ intervals using distance and time as explanatory variables; contralateral F1,136=67.14, ipsilateral F1,108=105.04). This reduction in forward walking velocity was dependent on the presentation distance of the mesh: crickets slowed down more during close as opposed to far presentation (contralateral F1,136=8.02, P<0.01; ipsilateral F1,108=6.31, P<0.05; Fig. 3C).
Phonotaxis did not substantially alter the responsiveness to the antennal stimulus. The time course of the responses to antennal stimulation differed between contralateral and ipsilateral trials. During ipsilateral stimulation, crickets maintained a low, constant forward speed (grey-shaded box in Fig. 3A) resembling the plateau-shaped response of spontaneously walking crickets (grey-shaded box in Fig. 2B). Curiously, following close ipsilateral antennal stimulation, crickets reduced their forward speed further (star in Fig. 3A), similar to close antennal stimulation in spontaneously walking crickets (star in Fig. 2B) before slowly accelerating to pre-stimulus conditions. In contralateral trials, the deceleration was more transient and the forward speed had already significantly increased during ongoing antennal stimulation (P<0.001, one-sided test comparing the ‘stim’ and ‘late stim’ intervals, i.e. the first and second half of the antennal stimulation period, close and far trials pooled).
Although the forward speed during antennal stimulation with a given presentation distance did not differ between the three experimental conditions (contralateral, ipsilateral and antennal only; Fig. 3C), systematic differences were observed during the ‘post’ interval following antennal stimulation (Fig. 3D). During and after retraction of the mesh, forward velocities generally remained lower than before antennal stimulation. This after-effect was significantly stronger after close antennal stimulation (ANOVA over ‘pre’ and ‘post’ intervals using distance and time as explanatory variables; contralateral: F1,136=13.21, P<0.001; ipsilateral: F1,108=8.61, P<0.01; Fig. 3D) and seemed more pronounced in ipsilateral compared with contralateral stimulation.
Phonotaxis is impaired during antennal stimulation
Following the observation that antennal stimuli had a strong effect on the crickets’ forward velocity even during phonotaxis, we investigated how antennal-evoked and auditory-evoked steering is integrated at the level of behaviour.
During unperturbed acoustic stimulation preceding far or close antennal stimulation, crickets responded with phonotactic steering toward the sound with similar turn amplitude: crickets turned right with 0.39 cm s−1 in contralateral trials, while they turned left with 0.56 cm s−1 in ipsilateral trials (Fig. 4, ‘pre’ interval, close and far trials pooled).
When presented with the mesh to their right side, walking crickets engaged in phonotaxis slowed down and orientated toward the antennal stimulus, similar to spontaneously walking crickets. During ipsilateral acoustic stimulation, crickets were already steering toward the side of the object and weakly reduced their steering velocity by 0.25 cm s−1 in close trials and by 0.13 cm s−1 in far trials (P<0.001, two-sided test comparing ‘pre’ and ‘stim’ intervals). In contralateral trials, antennal stimulation induced a strong shift in steering velocity away from the sound and toward the object (close: 0.35 cm s−1, far: 0.41 cm s−1; P<0.001 two-sided test comparing ‘pre’ and ‘stim’ intervals). As a consequence, phonotactic steering was abolished during contralateral antennal stimulation (Fig. 4B, ‘stim’ interval).
We found that in ipsilateral trials, crickets turned slightly more to the side of antennal and acoustic stimulation when the mesh was presented further away, with a response of 0.41 cm s−1 in far trials and 0.31 cm s−1 in close trials (P<0.1, paired two-sided test, comparing the ‘stim’ interval of close and far antennal stimulation). Thus, the orientation response was distance dependent, as observed in pure antennal stimulation trials. No distance dependence was observed in contralateral trials (P=0.23, paired two-sided test comparing close and far trials during ‘stim’ interval).
During close stimulation, some animals, i.e. 21% in ipsilateral trials and 3% in contralateral trials, initially displayed fast turns away from the approaching mesh – similar to what we had observed in spontaneously walking crickets – before they started exploring the object.
During the 5 s following antennal stimulation, phonotactic steering remained reduced compared with pre-antennal stimulation levels, possibly owing to continuing antennal contacts with the retracting mesh (Fig. 4, ‘post’ interval). To quantify responses to the retracting mesh as an after-effect of antennal stimulation, we performed a two-way ANOVA comparing the ‘pre’ and ‘post’ intervals using the presentation distance and time as explanatory variables. In crickets exposed to ipsilateral acoustic stimulation, the steering velocity after antennal stimulation was significantly reduced compared with pre-stimulation levels (F1,108=32.27, P<0.001). This reduction of phonotactic steering after antennal stimulation was more pronounced after close antennal stimulation (F1,108=4.20, P<0.05), i.e. it was distance dependent, consistent with the hypothesis that it results from interactions with the retracting mesh. Also, after contralateral stimulation there is a small, distance-dependent after-effect (F1,136=7.19, P<0.05).
These data demonstrate that the amplitude of the phonotactic steering velocity is generally smaller during antennal stimulation. The response to antennal stimulation depends on the presentation side and distance, and results in transiently reduced phonotaxis.
Phonotactic steering manoeuvres are partly suppressed during antennal stimulation
Reduced phonotactic steering could be the effect of linear superposition of conflicting steering manoeuvres and does not necessarily imply that during antennal stimulation the female cricket is unresponsive to the male's calling song. Goal-directed phonotaxis is accomplished by reactive steering manoeuvres in response to single chirps (Hedwig and Poulet, 2004). Hence, chirp-triggered lateral steering bouts indicate the cricket's engagement in phonotaxis and a reduction of those steering bouts may suggest that it no longer responds to the acoustic stimulus.
To estimate the magnitude of auditory steering over time, we averaged the chirp-triggered steering velocity to 10 consecutive chirps over 5 s long intervals covering the time before, during and after antennal stimulation (Fig. 5). In addition to the previously introduced ‘pre’, ‘stim’ and ‘post’ intervals, we analysed a ‘late stim’ interval covering the second half of antennal stimulation and a ‘late post’ interval covering the 5 s following the ‘post’ interval.
Fig. 5.
Reduction of chirp-triggered acoustic steering bouts during antennal stimulation. Median and IQR of the lateral steering velocity per chirp period averaged over 10 consecutive chirps within 5 s time intervals spanning the trial period. Five time intervals were chosen to cover the acoustic stimulation before, during and after antennal stimulation. These five intervals include the previously introduced ‘pre’, ‘stim’ and ‘post’ intervals. Two additional intervals, ‘late stim’ with seconds [5, 10) and ‘late post’ with seconds [15, 20), were introduced. Steering bouts to close and far stimulation are overlaid for ipsilateral (A) and contralateral trials (B). Acoustic and antennal stimulation during the analysed time period are schematised by speaker traces and mesh position. Grey shading highlights intervals during which the crickets were exposed to antennal stimulation. (A) Ipsilateral stimulation trials (n=28, 15 animals). (B) Contralateral stimulation trials (n=35, 20 animals).
During unperturbed phonotaxis, crickets showed characteristic lateral steering bouts toward the sound source that were coupled to individual chirps. We quantified the modulation amplitude of chirp-coupled steering bouts as the difference between the minimum and the maximum steering amplitude within one chirp period. During unperturbed phonotaxis, the median modulation amplitude of the steering bouts was 0.57 cm s−1 in contralateral trials and 0.67 cm s−1 in ipsilateral trials (Fig. 5A,B, ‘pre’ interval, mean of close and far trials). Under all experimental conditions, the sound-induced steering bouts were reduced during antennal stimulation compared with unperturbed phonotaxis (Fig. 5, grey-shaded ‘stim’ and ‘late stim’ intervals). The decrease between the ‘pre’ and ‘stim’ interval was more pronounced in close (reduction by 0.51 cm s−1 in contralateral and 0.25 cm s−1 in ipsilateral trials) as compared with far trials (decrease by 0.25 cm s−1 in contralateral and 0.19 cm s−1 in ipsilateral trials) and stronger during ipsilateral compared with contralateral stimulation. Acoustic steering bouts continued to be suppressed in the 5 s following close ipsilateral antennal stimulation, possibly owing to continuing contacts with the retracting mesh (Fig. 5A, ‘post’ interval). Suppression of phonotactic steering bouts during antennal stimulation suggests that motor responses triggered by antennal stimulation at least partly override phonotactic steering.
Analysis of leg kinematics in G. bimaculatus has revealed that adjustments of the leg movements during phonotactic steering are integrated into the regular stepping cycle (Witney and Hedwig, 2011). We noticed that the steering velocity showed regular oscillations during spontaneous walking (Fig. S2A,B) as well as during unperturbed phonotaxis (Fig. S2B), reflecting a regular tripod gait (Hedwig and Poulet, 2005; Witney and Hedwig, 2011). In all three experimental groups this stepping pattern appears to be disrupted during antennal stimulation (Fig. S2B, Movies 1–3).
No reduction of the response to extended antennal stimulation
The persistent impairment of phonotaxis during antennal stimulation raised the questions of whether crickets might adapt to a long-lasting antennal stimulus, for example, by reducing antennal exploration of the mesh, and whether they eventually return to phonotaxis. We therefore measured behavioural responses to ipsilateral and contralateral presentation of antennal and acoustic stimuli over a 30 s time period. The acoustic stimulus was presented alone for 10 s before the object was moved into antennal reach and kept there for the remaining 30 s.
Prior to antennal stimulation, crickets turned to the direction of the phonotactic stimulus with a similar median steering velocity across experimental conditions (contralateral: 0.52 cm s−1, ipsilateral: −0.78 cm s−1, close and far trials pooled; Fig. 6A,B, ‘pre’ interval). With the onset of antennal stimulation, crickets reduced their steering velocity toward the sound significantly by 0.63 cm s−1 in contralateral trials and by 0.54 cm s−1 in ipsilateral trials (both P<0.001, paired two-sided test comparing the ‘pre’ and ‘stim’ intervals, close and far trials pooled). The orientation response was accompanied by a significant reduction in median forward speed by 1.16 cm s−1 in contralateral and by 1.35 cm s−1 in ipsilateral trials (both: P<0.001, two-sided test, comparing the ‘pre’ and ‘stim’ intervals, close and far trials pooled).
Phonotactic steering remained impaired throughout the 30 s antennal stimulation period (Fig. 6A,B, grey-shaded box). To determine whether the steering or forward velocity varied with the presentation distance or the presentation time, we performed a two-way ANOVA over the entire stimulation period (six 5 s intervals marked above time axis in Fig. 6D). The orientation responses did not vary significantly with presentation time in contralateral (F1,224=0.329, P=0.57) or ipsilateral trials (F1,224=0.30, P=0.59). Also, the forward speed did not change significantly over the 30 s stimulation period (contralateral: F1,224=1.208, P=0.27, ipsilateral: F1,224=1.719, P=0.19). Crickets generally slowed down more in response to close compared with far antennal stimulation, which was more pronounced in contralateral (F1,224=41.626, P<0.001) compared with ipsilateral trials (F1,224=5.495, P<0.05). In turn, the presentation distance affected the orientation response in ipsilateral trials, where females made larger turns toward the mesh when presented at the far position (F1,224=14.66, P<0.005), but not in contralateral trials.
We evaluated video recordings of six animals to quantify when crickets made contacts with the mesh and plotted the contact times combined with the forward velocity. This revealed that crickets made repeated antennal contact with the mesh throughout the 30 s simulation period (Fig. 6C). The first antennal contact occurred on average 1.25 s (±0.25 s) earlier in close compared with far antennal stimulation trials, as the approaching mesh came within antennal reach before moving to its final position. For the same reason, antennal contact could be maintained for up to 2 s after close antennal stimulation. In far trials, contacts were primarily made with the right antenna, while additional contacts with the left antenna occurred in close trials. We found a negative correlation between the amount of antennal contacts a cricket made with the mesh and its forward velocity during far, but not close, presentation (Fig. 6D). Thus, in far trials the cricket slowed down more as the length of the antennal contacts increased (Fig. 6C).
Under our experimental conditions, phonotactic steering was suppressed and forward walking reduced for the entire 30 s of antennal stimulation. Responses to antennal stimulation did not vary significantly over the stimulation period, and crickets persistently made antennal contact with the mesh, indicating no behavioural adaptation to antennal stimulation.
DISCUSSION
The natural habitat of G. bimaculatus is grassland (Van Wyk and Ferguson, 1995). Female crickets performing phonotaxis when approaching a singing male thus have to navigate a cluttered terrain, where they encounter obstacles and predators. Walking crickets use their antennae to scan the space ahead, allowing them to detect objects along their path (Horseman et al., 1997). Further, antennal sensing plays an important role in the initiation of courtship following phonotaxis in two cricket species, G. bimaculatus and Teleogryllus oceanicus (Adamo and Hoy, 1994; Balakrishnan and Pollack, 1997). Therefore, sensory signals generated by antennal contact with objects in the cricket's path are likely of interest for a female cricket during phonotaxis. We provide a quantitative description of the responses to mechanosensory antennal stimulation generated by presenting an object to tethered walking female crickets. During antennal stimulation, crickets reduced their forward speed and turned toward the side of the object. Crickets engaged in phonotaxis responded to antennal stimulation in a similar manner, with phonotactic steering manoeuvres being suppressed for up to 30 s with no evidence for behavioural adaptation to the antennal stimulus. We found a negative correlation between the time crickets spent making contacts with the presented object and their forward walking speed. In short, crickets show immediate and persistent responses to antennal mechanosensory stimuli, which affect course control and suppress phonotaxis.
Antennal stimulation can elicit both exploratory and avoidance behaviour
Mechanosensory antennal sensing is involved in a variety of behaviours, ranging from flight-control over wall-following behaviour to escape responses (Comer et al., 1994; Hinterwirth and Daniel, 2010; Okada and Toh, 2006). Within those behaviours, it often leads to turning responses toward or away from the stimulus source.
Female G. bimaculatus in the field walk less straight and take longer to reach the sound source than crickets walking on a treadmill under laboratory conditions, a discrepancy that may result from rough terrain and obstacle negotiation during phonotaxis in the field (Hirtenlehner et al., 2014); similar observations were made in bushcrickets (von Helversen et al., 2001). Based on these findings, we expected that crickets perform transient turns away from an object in an attempt to bypass the obstacle. Surprisingly, in our paradigm crickets primarily slowed down and turned toward the object. These changes in walking behaviour, which could last for tens of seconds, were accompanied by an orientation of the cricket's head toward the object and antennal palpation. These observations suggest that the mechanosensory stimulus induced exploratory behaviour. However, some crickets responded to the approaching mesh with avoidance behaviour. It was more frequently observed in spontaneously walking females and in ipsilateral trials, where females already turned toward the approaching mesh. We hypothesise that these animals detected the movement of the approaching object and that this stimulus elicited avoidance behaviour. In G. bimaculatus, weak antennal contact with spider legs elicits primarily antennal search, whereas strong contact elicits avoidance behaviour (Okada and Akamine, 2012). These findings, together with observations of antennal use in cockroaches (Comer et al., 1994; Okada and Toh, 2004b, 2006; Stierle et al., 1994), indicate that behavioural responses to antennal stimulation depend on fine characteristics of the perceived stimulus.
Both exploratory and avoidance behaviour in response to mechanosensory antennal stimulation may be mediated by identified descending neurons, some of which have been shown to elicit turning upon current injection (Schöneich et al., 2011; Zorović and Hedwig, 2013).
Interaction of phonotaxis and antennal stimulation
The deceleration and orientation response to antennal stimulation impaired calling song tracking, temporarily overriding a robust behaviour such as phonotaxis. Behavioural studies in tethered crickets have shown that phonotaxis emerges from small reactive steering manoeuvres in response to single pulses and chirps (Hedwig and Poulet, 2004, 2005). Thus, a characteristic feature of phonotactic steering is that the temporal pattern of the acoustic stimulus is preserved in the motor output. We found that during antennal stimulation, phonotactic steering bouts were abolished and the regular stepping pattern was disrupted. This suggests that turns towards the antennal stimulus, in contrast to phonotactic steering, are not integrated into the regular walking pattern but rather initiate a different motor program: the animal stops and explores the object. In accordance with this, we found that when the mesh was presented alone, crickets slowed down and turned towards the mesh. Characteristic features of the response, such as the persistent reduction in forward speed and an additional deceleration after the retraction of the mesh, are preserved during ipsilateral acoustic stimulation. In contrast, during contralateral acoustic stimulation, the forward speed recovers already during antennal stimulation. This suggests that the behaviour during antennal stimulation is primarily governed by the sequence of antennal contacts, which in turn may be influenced by the recent mechanosensory experience of the animal but also by simultaneous acoustic stimulation. In trials with contralateral acoustic stimulation, crickets occasionally may be ‘pulled’ away from the antennal stimulus, make fewer antennal contacts with the object and, as a consequence, the temporal dynamics of the forward velocity differ from those in spontaneously walking crickets and in trials with ipsilateral acoustic stimulation.
By comparison, integration of phonotaxis with responses to visual stimuli shows different characteristics. In G. bimaculatus, orientation responses to a male's calling song and to a simultaneously presented black vertical stripe, an attractive visual stimulus, are additive (Böhm et al., 1991; Payne et al., 2010), and in bush crickets phonotactic tracking improves in the presence of stationary visual cues (von Helversen and Wendler, 2000). The apparently different mechanism of integration of phonotaxis with responses to antennal stimulation may be explained by the qualitatively different requirements of responses to mechanosensory and visual stimuli. Visual, like acoustic signals, act on a long range and do not necessarily require immediate action. In contrast, antennae are contact sensors, providing information about the animal's close-range environment. Consequently, antennal stimuli may often require an immediate response, for example, during obstacle negotiation, mate recognition or predator avoidance.
Our data show that mechanosensory antennal stimuli are important to female crickets during phonotaxis. This has implications for the design of phonotaxis experiments and may lead to differences in phonotactic behaviour across laboratory paradigms.
Why do we not see adaptation to the antennal stimulus?
We were surprised to find sustained impairment of phonotaxis even over a 30 s antennal stimulation period. Okada and Toh (2004a) demonstrated in free-walking, blinded cockroaches that antennal contact marks the beginning of a motor sequence, where detection of an object induces changes in antennal movement, followed by head turning, approach and climbing attempts.
Under natural conditions, walking manoeuvres, head movements and antennal movements jointly control the distance and orientation of the antennae relative to an object. Changes in any of these movement patterns have an impact on antennal sensing. When feedback of the animal's behaviour onto its own sensory experience is largely removed, as is the case in our quasi open-loop antennal stimulation paradigm, we may only see the beginning of this behavioural sequence. Instead of a transient turn initiated by antennal contact, after which the animal reaches the object and terminates the approach manoeuvre, the behavioural sequence stalls as the animal persistently aims to reach the object. Under open-loop conditions, cockroaches walking on a trackball turn toward a rod brought into reach of one antennae for up to 30 s (Okada and Toh, 2006). Interpreting the antennal stimulation-evoked orientation toward the object as an attempt to approach the object would also explain why turn responses were more pronounced when the object was far away from the cricket: the animal might attempt to approach the more distant object in order to further explore it. Consistent with the hypothesis that turns toward the mesh were aimed at exploring the object, we observed repeated contacts with both antennae during close antennal stimulation.
Where do antennal and phonotactic pathways converge?
Female crickets sense the male's calling song via ears on their front legs, from where auditory afferents project to the thoracic ganglion (Eibl and Huber, 1979; Esch et al., 1980). Ascending interneurons convey the signal to the brain, where the pattern is processed by a series of interneurons in a frontal auditory neuropil of the protocerebrum and in the lateral accessory lobe (Kostarakos and Hedwig, 2012; Schöneich et al., 2015; Zorović and Hedwig, 2011). From the lateral accessory lobe, descending interneurons, whose activity weakly reflects the calling song's pattern as well as walking velocities, project to thoracic ganglia (Zorović and Hedwig, 2013).
The pathway for mechanical information is very different. Mechanosensory afferents coming from the cricket's antennae enter the brain via the antennal nerve and project to the deutocerebrum and the subesophageal ganglion (Rospars, 1988; Staudacher and Schildberger, 1999; Yoritsune and Aonuma, 2012). From the deutocerebrum, giant descending interneurons convey information to the ventral nerve cord (Gebhardt and Honegger, 2001; Schöneich et al., 2011). Three of these interneurons respond to both visual and mechanosensory stimuli, and one has been shown to elicit walking bouts and contralateral steering upon current injection (Zorović and Hedwig, 2013). These findings are consistent with a fast descending escape circuit that receives, amongst others, mechanosensory input from the antennae. Antennal contact with an approaching, but not a stationary, object might activate this circuit to elicit the stereotypic avoidance manoeuvre observed in a subset of animals. A descending neuron, which responds to antenno-mechanosensory and visual stimuli, may participate in the detection of objects in the cricket's walking path (Gebhardt and Honegger, 2001), suggesting involvement in exploration or obstacle negotiation. Notably, antennal interneurons so far described do not receive strong auditory inputs (Zorović and Hedwig, 2013).
Therefore, antennal mechanosensory and auditory stimuli are processed in different brain areas, suggesting that early sensorimotor processing of these signals is performed by separate pathways and convergence might only occur in the thoracic ganglia. Alternatively, acoustic stimulation may influence antennal sensing by changing the antennal search patterns. In cockroaches, attractive and aversive odours have been shown to have differential effects on both locomotion and antennal search patterns (Nishiyama et al., 2007).
To further probe the neural computations underlying the processing of antennal stimuli and how responses to antennal and acoustic stimuli are integrated, our behavioural paradigm may be combined with neurophysiological techniques as well as video tracking of antennal movements.
Suppression of phonotaxis by antennal stimulation as a model system for studying action selection and active sensing
Studies on multimodal integration have focused on understanding the mechanisms underlying integration of cross-modal stimuli (Stein and Stanford, 2008). Integrating cross-modal stimuli increases reliability of the extracted information about the object or event. We looked at bimodal integration in a different context: an animal is confronted with two independent stimuli that affect the same behaviour, i.e. course control. In this case, processing of two sensory inputs needs to select the appropriate behavioural response and ensure a coordinated motor output.
We found that antennal mechanosensory stimuli suppress phonotaxis. Suppression or interruption of an ongoing behaviour by a novel stimulus is a common phenomenon. For example, stimulation of the cerci, another mechanosensory organ, interrupts singing in male crickets (Hedwig, 2000; Jacob and Hedwig, 2015). In feeding crayfish, the escape response is suppressed by a mechanism called ‘tonic inhibition’, which has been suggested as a mechanism for action selection (Krasne and Lee, 1988; Vu and Krasne, 1992; Vu et al., 1993). Hierarchical suppression has also been proposed as a mechanism for generating behavioural sequences (Seeds et al., 2014).
Although crickets responded to a conspecific male's calling song reliably with phonotaxis, both exploration or avoidance behaviour was observed during antennal stimulation. This was likely a consequence of letting the animal generate the mechanosensory stimulus rather than imposing controlled antennal movements and contacts. A situation such as this, in which an animal controls stimulus intensity and frequency, has been classified as active sensing (Prescott et al., 2011; Staudacher et al., 2005). Examples of active touch sensing in mammals are whisking in rodents (Grant et al., 2009) and palpation with the hand in capuchin monkeys (Visalberghi et al., 2009). Active touch sensing is also found in many insects (Comer and Baba, 2011), especially in the context of navigation (Harley et al., 2009; Okada and Toh, 2004a, 2006; Schütz and Dürr, 2011). The interaction of the crickets' active antennal movements and the experimentally controlled positioning of the mesh resulted in slightly different tactile stimuli possibly activating different motor programs. This variability observed in our paradigm could be exploited to study selection and coordination of different motor programs in the context of active sensing.
Acknowledgements
We thank Steve Ellis and Glen Harrison for excellent support, providing essential mechanical devices of the stimulation apparatus and the electronic control circuits. We also thank Pedro Jacob, Tim Bailey and the anonymous reviewers for constructive comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
H.H. and B.H. designed the experiments and interpreted the results. H.H. collected and analysed the data. H.H. and B.H. wrote the manuscript.
Funding
This work was supported by the Howard Hughes Medical Institute's Janelia Graduate Scholars Program. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.141606.supplemental
References
- Adamo S. A. and Hoy R. R. (1994). Mating behaviour of the field cricket Gryllus bimaculatus and its dependence on social and environmental cues. Anim. Behav. 47, 857-868. 10.1006/anbe.1994.1117 [DOI] [Google Scholar]
- Balakrishnan R. and Pollack G. (1997). The role of antennal sensory cues in female responses to courting males in the cricket Teleogryllus oceanicus. J. Exp. Biol. 200, 511-522. [DOI] [PubMed] [Google Scholar]
- Böhm H., Schildberger K. and Huber F. (1991). Visual and acoustic course control in the cricket Gryllus bimaculatus. J. Exp. Biol. 159, 235-248. [Google Scholar]
- Comer C. and Baba Y. (2011). Active touch in orthopteroid insects: behaviours, multisensory substrates and evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 3006-3015. 10.1098/rstb.2011.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer C. M., Mara E., Murphy K. A., Getman M. and Mungy M. C. (1994). Multisensory control of escape in the cockroach Periplaneta americana. II. Patterns of touch-evoked behavior. J. Comp. Physiol. A 174, 13-26. 10.1007/BF00192002 [DOI] [Google Scholar]
- Eibl E. and Huber F. (1979). Central projections of tibial sensory fibers within the three thoracic ganglia of crickets (Gryllus campestris L., Gryllus bimaculatus DeGeer). Zoomorphologie 92, 1-17. 10.1007/BF00999832 [DOI] [Google Scholar]
- Esch H., Huber F. and Wohlers D. W. (1980). Primary auditory neurons in crickets: physiology and central projections. J. Comp. Physiol. A 137, 27-38. 10.1007/BF00656914 [DOI] [Google Scholar]
- Gebhardt M. and Honegger H. W. (2001). Physiological characterisation of antennal mechanosensory descending interneurons in an insect (Gryllus bimaculatus, Gryllus campestris) brain. J. Exp. Biol. 204, 2265-2275. [DOI] [PubMed] [Google Scholar]
- Gerhardt H. C. and Huber F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- Grant R. A., Mitchinson B., Fox C. W. and Prescott T. J. (2009). Active touch sensing in the rat: anticipatory and regulatory control of whisker movements during surface exploration. J. Neurophysiol. 101, 862-874. 10.1152/jn.90783.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. M., English B. A. and Ritzmann R. E. (2009). Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J. Exp. Biol. 212, 1463-1476. 10.1242/jeb.028381 [DOI] [PubMed] [Google Scholar]
- Hedwig B. (2000). Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J. Neurophysiol. 83, 712-722. [DOI] [PubMed] [Google Scholar]
- Hedwig B. and Poulet J. F. A. (2004). Complex auditory behaviour emerges from simple reactive steering. Nature 430, 781-785. 10.1038/nature02787 [DOI] [PubMed] [Google Scholar]
- Hedwig B. and Poulet J. F. A. (2005). Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J. Exp. Biol. 208, 915-927. 10.1242/jeb.01452 [DOI] [PubMed] [Google Scholar]
- Hirtenlehner S. and Römer H. (2014). Selective phonotaxis of female crickets under natural outdoor conditions. J. Comp. Physiol. A 200, 239-250. 10.1007/s00359-014-0881-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtenlehner S., Römer H. and Schmidt A. K. D. (2014). Out of phase: relevance of the medial septum for directional hearing and phonotaxis in the natural habitat of field crickets. J. Comp. Physiol. A 200, 139-148. 10.1007/s00359-013-0869-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterwirth A. J. and Daniel T. L. (2010). Antennae in the hawkmoth Manduca sexta (Lepidoptera, Sphingidae) mediate abdominal flexion in response to mechanical stimuli. J. Comp. Physiol. A 196, 947-956. 10.1007/s00359-010-0578-5 [DOI] [PubMed] [Google Scholar]
- Horseman B. G., Gebhardt M. J. and Honegger H.-W. W. (1997). Involvement of the suboesophageal and thoracic ganglia in the control of antennal movements in crickets. J. Comp. Physiol. A 181, 195-204. 10.1007/s003590050106 [DOI] [Google Scholar]
- Jacob P. F. and Hedwig B. (2015). The impact of cercal air currents on singing motor pattern generation in the cricket (Gryllus bimaculatus DeGeer). J. Neurophysiol. 114, 2649-2660. 10.1152/jn.00669.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K. and Hedwig B. (2012). Calling song recognition in female crickets: temporal tuning of identified brain neurons matches behavior. J. Neurosci. 32, 9601-9612. 10.1523/JNEUROSCI.1170-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne F. B. and Lee S. C. (1988). Response-dedicated trigger neurons as control points for behavioral actions: selective inhibition of lateral giant command neurons during feeding in crayfish. J. Neurosci. 8, 3703-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart T., Hodel B. and Valenzuela I. (1984). The physiology of the cricket's compound eye with particular reference to the anatomically specialized dorsal rim area J. Comp. Physiol. A 155, 289-296. 10.1007/BF00610582 [DOI]
- Murphey R. K. and Zaretsky M. D. (1972). Orientation to calling song by female crickets, Scapsipedus marginatus (Gryllidae). J. Exp. Biol. 56, 335-352. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Okada J. and Toh Y. (2007). Antennal and locomotor responses to attractive and aversive odors in the searching cockroach. J. Comp. Physiol. A 193, 963-971. 10.1007/s00359-007-0249-3 [DOI] [PubMed] [Google Scholar]
- Okada J. and Akamine S. (2012). Behavioral response to antennal tactile stimulation in the field cricket Gryllus bimaculatus. J. Comp. Physiol. A 198, 557-565. 10.1007/s00359-012-0729-y [DOI] [PubMed] [Google Scholar]
- Okada J. and Toh Y. (2004a). Antennal system in cockroaches: a biological model of active tactile sensing. Int. Congr. Ser. 1269, 57-60. 10.1016/j.ics.2004.05.014 [DOI] [Google Scholar]
- Okada J. and Toh Y. (2004b). Spatio-temporal patterns of antennal movements in the searching cockroach. J. Exp. Biol. 207, 3693-3706. 10.1242/jeb.01201 [DOI] [PubMed] [Google Scholar]
- Okada J. and Toh Y. (2006). Active tactile sensing for localization of objects by the cockroach antenna. J. Comp. Physiol. A 192, 715-726. 10.1007/s00359-006-0106-9 [DOI] [PubMed] [Google Scholar]
- Payne M., Hedwig B. and Webb B. (2010). Multimodal predictive control in crickets. In From Animals to Animats 11. Volume 6226 of the series Lecture Notes in Computer Science (ed. Doncieux S., Girard B., Guillot A., Hallam J., Meyer J.-A. and Mouret J.-B.), pp. 167-177. Berlin: Springer. [Google Scholar]
- Prescott T. J., Diamond M. E. and Wing A. M. (2011). Active touch sensing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2989-2995. 10.1098/rstb.2011.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen J. (1913). Über die Anlockung des Weibchens von Gryllus campestris L. durch telephonisch übertragene Stridulationslaute des Männchens. Pfluegers Arch. Ges. Physiol. 193-200. [Google Scholar]
- Ritzmann R. E., Harley C. M., Daltorio K. A., Tietz B. R., Pollack A. J., Bender J. A., Guo P., Horomanski A. L., Kathman N. D., Nieuwoudt C. et al. (2012). Deciding which way to go: how do insects alter movements to negotiate barriers? Front. Neurosci. 6, 97 10.3389/fnins.2012.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz R., Bretman A., Slate J., Walling C. A. and Tregenza T. (2010). Natural and sexual selection in a wild insect population. Science 328, 1269-1272. 10.1126/science.1188102 [DOI] [PubMed] [Google Scholar]
- Rospars J. P. (1988). Structure and development of the insect antennodeutocerebral system. Int. J. Insect Morphol. Embryol. 17, 243-294. 10.1016/0020-7322(88)90041-4 [DOI] [Google Scholar]
- Schmitz B., Scharstein H. and Wendler G. (1982). Phonotaxis in Gryllus campestris L. (Orthoptera, Gryllidae) – I. Mechanism of acoustic orientation in intact female crickets. J. Comp. Physiol. A 148, 431-444. 10.1007/BF00619782 [DOI] [Google Scholar]
- Schöneich S., Schildberger K. and Stevenson P. A. (2011). Neuronal organization of a fast-mediating cephalothoracic pathway for antennal-tactile information in the cricket (Gryllus bimaculatus DeGeer). J. Comp. Neurol. 519, 1677-1690. 10.1002/cne.22594 [DOI] [PubMed] [Google Scholar]
- Schöneich S., Kostarakos K. and Hedwig B. (2015). An auditory feature detection circuit for sound pattern recognition. Sci. Adv. 1, e1500325 10.1126/sciadv.1500325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz C. and Dürr V. (2011). Active tactile exploration for adaptive locomotion in the stick insect. Philos. Trans. R. Soc. B Biol. Sci. 366, 2996-3005. 10.1098/rstb.2011.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds A. M., Ravbar P., Chung P., Hampel S., Midgley F. M. Jr, Mensh B. D. and Simpson J. H. (2014). A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. Elife 3, e02951 10.7554/eLife.02951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W. (1988). Male size, mating potential and lifetime reproductive success in the field cricket, Gryllus bimaculatus (De Geer). Anim. Behav. 36, 372-379. 10.1016/S0003-3472(88)80008-3 [DOI] [Google Scholar]
- Staudacher E. and Schildberger K. (1999). A newly described neuropile in the deutocerebrum of the cricket: antennal afferents and descending interneurons. Zoology 102, 212-226. [Google Scholar]
- Staudacher E. M., Gebhardt M. and Dürr V. (2005). Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv. Insect Phys. 32, 49-205. 10.1016/s0065-2806(05)32002-9 [DOI] [Google Scholar]
- Stein B. E. and Stanford T. R. (2008). Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 9, 255-266. 10.1038/nrn2331 [DOI] [PubMed] [Google Scholar]
- Stierle I. E., Getman M. and Comer C. M. (1994). Multisensory control of escape in the cockroach Periplaneta americana. I. Initial evidence from patterns of wind-evoked behavior. J. Comp. Physiol. A 174, 1-11. 10.1007/BF00192001 [DOI] [Google Scholar]
- Van Wyk J. W. and Ferguson J. W. H. (1995). Communicatory constraints on field crickets Gryllus bimaculatus calling at low ambient temperatures. J. Insect Physiol. 41, 837-841. 10.1016/0022-1910(95)00055-Y [DOI] [Google Scholar]
- Visalberghi E., Addessi E., Truppa V., Spagnoletti N., Ottoni E., Izar P. and Fragaszy D. (2009). Selection of effective stone tools by wild bearded Capuchin monkeys. Curr. Biol. 19, 213-217. 10.1016/j.cub.2008.11.064 [DOI] [PubMed] [Google Scholar]
- von Helversen D. and Wendler G. (2000). Coupling of visual to auditory cues during phonotactic approach in the phaneropterine bushcricket Poecilimon affinis. J. Comp. Physiol. A 186, 729-736. 10.1007/s003590000126 [DOI] [PubMed] [Google Scholar]
- von Helversen D., Schul J. and Kleindienst H.-U. (2001). Male recognition mechanism for female responses implies a dilemma for their localisation in a phaneropterine bushcricket. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 186, 1153-1158. 10.1007/s003590000167 [DOI] [PubMed] [Google Scholar]
- Vu E. T. and Krasne F. B. (1992). Evidence for a computational distinction between proximal and distal neuronal inhibition. Science 255, 1710-1712. 10.1126/science.1553559 [DOI] [PubMed] [Google Scholar]
- Vu E. T., Lee S. C. and Krasne F. B. (1993). The mechanism of tonic inhibition of crayfish escape behavior: distal inhibition and its functional significance. J. Neurosci. 13, 4379-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T. and Thorson J. (1989). Phonotactic behaviour of walking crickets. In Cricket Behavior and Neurobiology (ed. Huber F., Moore T. and Loher W.), pp. 301-339. Ithaca, NY: Cornell University Press. [Google Scholar]
- Witney A. G. and Hedwig B. (2011). Kinematics of phonotactic steering in the walking cricket Gryllus bimaculatus (de Geer). J. Exp. Biol. 214, 69-79. 10.1242/jeb.044800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoritsune A. and Aonuma H. (2012). The anatomical pathways for antennal sensory information in the central nervous system of the cricket, Gryllus bimaculatus. Invertebr. Neurosci. 12, 103-117. 10.1007/s10158-012-0137-6 [DOI] [PubMed] [Google Scholar]
- Zorović M. and Hedwig B. (2011). Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J. Neurophysiol. 105, 2181-2194. 10.1152/jn.00416.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorović M. and Hedwig B. (2013). Descending brain neurons in the cricket Gryllus bimaculatus (de Geer): auditory responses and impact on walking. J. Comp. Physiol. A 199, 25-34. 10.1007/s00359-012-0765-7 [DOI] [PubMed] [Google Scholar]