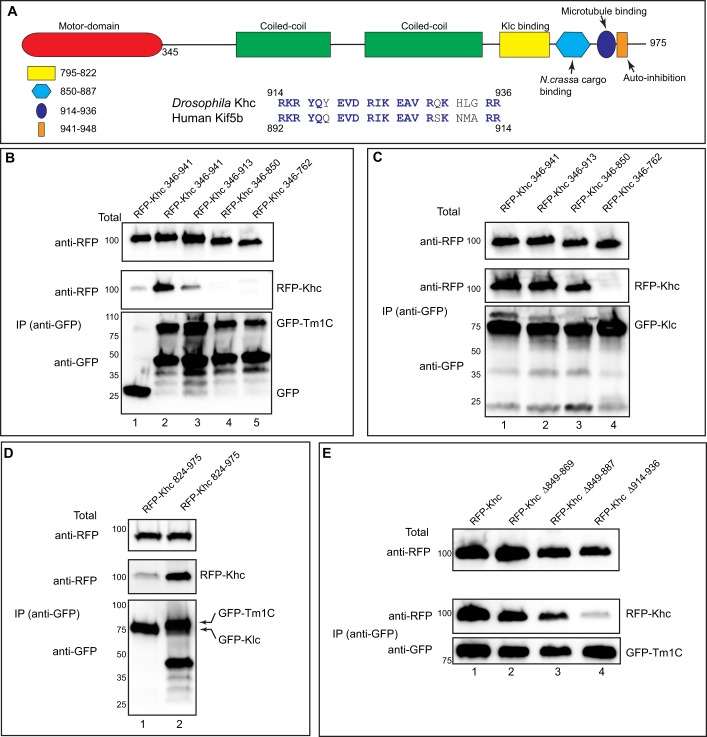
Fig. 2.
Tm1C binds to a C-terminal domain within Khc. (A) Domain structure of Khc. The following features are indicated: motor domain (red), coiled-coil domains required for dimerization of Khc (green), the Klc-binding site (yellow), a region important for cargo binding in N. crassa (light blue), an ATP-independent microtubule-binding region (blue) and an auto-inhibitory domain (dark blue oval). Also shown is an alignment of amino acids 914 to 936 of Khc with Kif5b, the human homolog of Khc. (B) A co-immunoprecipitation experiment using various RFP–Khc truncation constructs with full-length GFP–Tm1C. The residues included in the truncations are indicated. Lysates were prepared and the GFP-tagged proteins were immunoprecipitated. The co-precipitating proteins and the total fraction were analyzed by blotting using the indicated antibodies. (C) The same truncation constructs used in the previous panel were co-transfected with full-length GFP–Klc. The experiment was performed as in B. (D) A co-immunoprecipitation experiment using a small RFP–Khc construct and either GFP–Klc (lane 1) or Tm1C (lane 2). The immunoprecipitation was performed as described for B and analyzed using the indicated antibodies. (E) A co-immunoprecipitation experiment using GFP–Tm1C and either full-length RFP–Khc or RFP–Khc containing the indicated deletions. The co-precipitation was performed as described for B and analyzed by blotting using the indicated antibodies.