Abstract
β-Galactosidase from Streptococcus thermophilus was overexpressed in a food-grade organism, Lactobacillus plantarum WCFS1. Laboratory cultivations yielded 11,000 U of β-galactosidase activity per liter of culture corresponding to approximately 170 mg of enzyme. Crude cell-free enzyme extracts obtained by cell disruption and subsequent removal of cell debris showed high stability and were used for conversion of lactose in whey permeate. The enzyme showed high transgalactosylation activity. When using an initial concentration of whey permeate corresponding to 205 g L−1 lactose, the maximum yield of galacto-oligosaccharides (GOS) obtained at 50°C reached approximately 50% of total sugar at 90% lactose conversion, meaning that efficient valorization of the whey lactose was obtained. GOS are of great interest for both human and animal nutrition; thus, efficient conversion of lactose in whey into GOS using an enzymatic approach will not only decrease the environmental impact of whey disposal, but also create additional value.
Keywords: Whey permeate, Galacto-oligosaccharides (GOS), β-galactosidase, Prebiotics, Transgalactosylation
1. Introduction
Cheese whey is the most significant waste from the dairy industry and can cause significant environmental pollution problems [1]. This by-product is generated upon coagulation of caseins in cheese making, and corresponds to 85–95% of the milk volume. The liquid whey retains about 55% of milk nutrients [2], of which lactose (4.5 5% w/v) is the most abundant. Removal of valuable whey proteins leaves whey permeate, which can contain up to 85% lactose based on dry matter. Several studies have shown that lactose is largely responsible for the high biochemical oxygen demand (BOD) and chemical oxygen demand (COD) of whey [1]. Annual world-wide cheese whey production amounts to over 160 million tons per year, corresponding to approximately 6 million tons of lactose [3,4]. Whey poses significant challenges to the dairy industry’s environmental protection strategies. High production of cheese whey and whey permeate as well as their high environmental impact and nutritional content make them an important subject for careful valorization studies. Different ways of whey valorization have been investigated, both decreasing environmental impact and exploring the possibilities of reusing nutrients [5].
Due to the abundant amount of lactose in whey, one approach for whey valorization that has attracted increasing attention is the bioconversion of lactose (β-d-Galactose-(1→4)-d-Glucose) to valuable products using β-galactosidases. β-Galactosidases (β-Gal; EC 3.2.1.23) catalyze both the hydrolysis and transgalactosylation of β-d-galactopyranosides, including lactose [6–8] and are found widespread in nature. They catalyze the hydrolysis of lactose and related compounds, and are thus used in dairy industry to remove lactose from various products. These enzymes often also show transgalactosylation activity [6,9], which is of interest because the resulting galacto-oligosaccharides (GOS) are non-digestible carbohydrates with known prebiotic activity. GOS generally comprise one or more galactose units that are typically linked to a terminal glucose. The degree of polymerization of GOS can vary quite markedly, ranging from 2 to 8 monomeric units. GOS are thus complex mixtures of different oligosaccharides, and the spectrum of the oligosaccharides making up these mixtures strongly depends on the source of the β-galactosidase used for the biocatalytic reaction as well as on the conversion conditions used in their production. GOS can serve as fermentable substrates for certain members of the gut microbiota, and have been found to modulate the colonic flora by stimulation of beneficial bacteria, such as bifidobacteria and lactobacilli, and inhibition of less desirable bacteria [10–12]. Potential health benefits of GOS include reduction of intestinal disturbances (constipation and diarrhea), cardiovascular disease and intestinal cancer, increased absorption and retention of several minerals, particularly magnesium, calcium, and iron, modulation of immune responses, as well as reduction of serum cholesterol levels [13–18]. Because of these benefits, GOS are of great interest for both human and animal nutrition. Furthermore, GOS are of special interest because of the presence of structurally related oligosaccharides in human breast milk [14,19,20].
In this paper, we describe the use of β-galactosidase from Streptococcus thermophilus, which is recombinantly produced in food-grade Lactobacillus plantarum, for the efficient conversion of lactose in whey to obtain GOS. Conversion of an important food waste into more valuable products is advantageous not only for the environment but also for sustainable economics.
2. Materials and methods
2.1. Chemicals and enzymes
All chemicals and enzymes were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise and were of the highest quality available. All restriction enzymes, T4 DNA ligase, and corresponding buffers were from Fermentas (Vilnius, Lithunia).
2.2. Bacterial strains and culture conditions
Streptococcus salivarius subsp. thermophilus DSM 20259 (synonym S. thermophilus) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). L. plantarum WCFS1, isolated from human saliva as described by Kleerebezem et al. [21], was originally obtained from NIZO Food Research (Ede, The Netherlands) and maintained in the culture collection of the Norwegian University of Life Sciences, Ås, Norway. Escherichia coli DH5α (New England Biolabs, Frankfurt am Main, Germany) was used in the transformation experiments involving the subcloning of DNA fragments. S. thermophilus and L. plantarum were cultivated in M-17 broth and in MRS media, respectively, at 37°C without agitation. E. coli NEB5α (New England Biolabs, Frankfurt am Main, Germany) was grown at 37°C in Luria–Bertani (LB) medium with shaking at 120 rpm. When needed, erythromycin was supplemented to media in concentrations of 5 μg/mL for Lactobacillus or 200 μg/mL for E. coli, whereas ampicillin was used at 100 μg/mL for E. coli.
2.3. Construction of β-galactosidase expression vectors
The lacZ gene, which encodes for a β-galactosidase from S. thermophilus DSM2 0259 (NCBI Reference No. CP000419), was amplified using proof-reading Phusion polymerase with the primer pair FwdStNcoI (5′-GCGGCCATGGACATGACTGAAAAAATTCAAAC-3′) and RevStXhoI (5′-GGCGCTCGAGCTAATTTAGTGGTTCAATCATG-3′). The forward primer, FwdStNcoI, contains an NcoI restriction site and the reverse primer, RevStXhoI, includes an XhoI recognition site (underlined). Genomic DNA of S. thermophilus isolated according to a previously described procedure [22] was used as template for the PCR reaction. The initial denaturation step at 98°C for 3 min was follow by 30 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s and extension at 72°C for 40 s, followed by a final extension step at 72°C for 5 min. The amplified gene was digested with NcoI and XhoI after which the PCR product was purified using the Wizard SV Gel and PCR Clean-up system kit (Promega, Madison, WI). The PCR fragment was subcloned into the pJET1.2 plasmid (CloneJET PCR cloning kit, Fermentas), and E. coli NEB5α was used as a host for obtaining the plasmids in sufficient amounts. The sequence of the insert was confirmed by DNA sequencing performed by a commercial provider (Microsynth, Vienna, Austria). The gene fragment of lacZ was then cloned into the expression vector pSIP409, which employs regulatory elements of the sakacin P operon of Lactobacillus sakei [23,24], using NcoI and XhoI cloning sites, resulting in the plasmid p409lacZSt. The constructed plasmid was transformed into electrocompetent cells of L. plantarum WCFS1 according to the protocol of Aukrust and Blom [25].
2.4. Expression of recombinant β-galactosidase
For the heterologous overexpression of the lacZ gene from S. thermophilus, an overnight culture (~16 h) of L. plantarum WCFS1 harboring the expression plasmid p409lacZSt was used to inoculate 50 mL of fresh MRS medium containing erythromycin, with a starting OD600 of ~0.1. The culture was incubated at 30°C without agitation and the cells were induced at an OD600 of 0.3 by adding 25 ng/mL of the inducing peptide pheromone IP-673 [26]. Cells were harvested at an OD600 of 1.8–2.0, washed twice using 50 mM sodium phosphate buffer, pH 6.5 and resuspended in 1 mL (2% of the original culture volume) of the same buffer. Cells were disrupted in a bead beating homogenizer using 1 g of glass bead (Precellys 24; PEQLAB, Germany). Cell-free extracts were obtained after a centrifugation step at 9000g for 15 min at 4°C.
2.5. Fermentation of recombinant L. plantarum
L. plantarum WCFS1 harboring p409lacZSt was cultivated in 1-L fermentations to obtain sufficient amounts of LacZ. The cultivation conditions and the induction protocol were identical to those described above for small-scale cultivations. Expression of lacZ was induced at OD600 of 0.3, the cells were harvested at OD600 ~6 and washed twice with 50 mM sodium phosphate buffer, pH 6.5 and the cells were then disrupted by passing the 20 mL suspension 3 times through a French press (AMINCO, Silver Spring, MD) with an applied pressure of 1000 psi. Cell debris was removed by centrifugation (25,000g, 20 min, 4°C). The lysate (crude extract) was then stored at −20°C.
2.6. Gel electrophoresis analysis
The cell-free extracts were analyzed by SDS-PAGE using the Phast System with precast gels (Pharmacia Biotech, Uppsala, Sweden). The enzyme preparation was diluted to 1 mg protein mL−1 and mixed with an equal volume of 2×Laemmli buffer, followed by incubation at 90°C for 5 min. Protein bands were visualized by staining with Bio-safe Coomassie (Bio-Rad). Unstained Precision plus Protein Standard (Bio-Rad) was used as mass marker.
2.7. β-Galactosidase assays
The measurement of β-galactosidase activity using o-nitrophenyl-β-d-galactopyranoside (oNPG) or lactose as the substrates was carried out as previously described [27]. When chromogenic oNPG was used as the substrate, the reaction was initiated by adding 20 μL of enzyme solution to 480 μL of 22 mM oNPG in 50 mM sodium phosphate buffer (pH 6.5) and stopped after 10 min of incubation at 30°C by adding 750 μL of 0.4 M Na2CO3. The release of o-nitrophenol (oNP) was measured by determining the absorbance at 420 nm. One unit of oNPG activity was defined as the amount of enzyme releasing 1 μmol of oNP per minute under the described conditions.
When lactose was used as the substrate, 20 μL of enzyme solution was added to 480 μL of a 600 mM lactose solution in 50 mM sodium phosphate buffer, pH 6.5. After 10 min of incubation at 30°C, the reaction was stopped by heating the reaction mixture at 99°C for 5 min. The reaction mixture was cooled to room temperature, and the release of D-glucose was determined using the test kit from Megazyme. One unit of lactase activity was defined as the amount of enzyme releasing 1 μmol of d-glucose per minute under the given conditions.
2.8. Temperature dependence of stability
The catalytic stability of crude recombinant β-galactosidase from S. thermophilus overexpressed in L. plantarum was determined by incubating the enzyme in 50 mM phosphate buffer or in whey permeate solution, pH 6.5 at 37°C and 50°C and by subsequent measurements of the remaining enzyme activity (A) at various time points (t) using the standard oNPG assay. Residual activities (At/A0, where At is the activity measured at time t and A0 is the initial activity) were plotted versus the incubation time. The inactivation constants kin were obtained by linear regression of ln(activity) versus time. The half-life values of thermal inactivation t1/2 were calculated using t1/2 = ln 2/kin [28].
2.9. Steady-state kinetic measurements
A small portion of the crude extract was used to purify the β-galactosidase to homogeneity by affinity chromatography using p-aminobenzyl 1-thio-β-d-galactopyranoside immobilized onto cross-linked 4% beaded agarose (Sigma), as previously described [27]. Steady-state kinetic data for the substrates lactose or oNPG were obtained at 30°C in 50 mM sodium phosphate buffer, pH 6.5, with concentrations ranging from 0 to 600 mM for lactose and from 0 to 22 mM for oNPG. The kinetic parameters were calculated using nonlinear regression, fitting the observed data to the Michaelis-Menten equation using SigmaPlot (SPSS, Chicago, IL).
2.10. Determination of protein concentration
Protein concentrations were determined by the method of Bradford [29] using bovine serum albumin as the standard.
2.11. Lactose hydrolysis and galacto-oligosaccharides synthesis
Batch conversion reactions were carried out with crude recombinant β-galactosidase from S. thermophilus using whey permeate powder (with approximately 65% of the dry matter being lactose) as the source of lactose. The influence of process parameters such as temperature (37°C, 50°C) and substrate concentration (50 and 200 g L−1 lactose) on the reaction was also studied. The substrate solution was prepared in 50 mM sodium phosphate buffer pH 6.5, containing 10 mM MgCl2. Agitation during these conversions was applied at 300 rpm using a thermomixer (Eppendorf, Hamburg, Germany).
2.12. Analysis of mono- and oligosaccharides
The carbohydrate composition in reaction mixtures was analysed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD), which was carried out on a Dionex DX-500 system consisting of a GP50 gradient pump, an ED 40 electrochemical detector with a gold working electrode and an Ag/AgCl reference electrode, and Chromeleon version 6.5 (Dionex Corp., Sunnyvale, CA). All eluents were degassed by flushing with helium for 30 min. Separations were performed at room temperature on a CarboPac PA-1 column (4 mm × 250 mm) connected to a CarboPac PA-1 guard column (Dionex) [30]. Separation of d-glucose, d-galactose, lactose and allolactose (β-d-Galp-(1→6)-d-Glc) was carried out with an isocratic run (45 min) with 15 mM NaOH at 1.0 mL min−1, followed by 25 min elution with 100 mM NaOH. For separation of GOS, eluents A (100 mM NaOH) and B (100 mM NaOH and 150 mM NaOAc) were mixed to form the following gradient: 98% A from 0 to 10 min, 98% A to 52% A from 10 to 40 min, and then 52% A for another 5 min. The column was washed with 20% B for 10 min and re-equilibrated for 15 min with the starting conditions of the employed gradient. Galacto-oligosaccharide standards of β-d-Galp-(1 → 3)-d-Glc, β-d-Galp-(1 → 6)-d-Glc, β-d-Galp-(1 → 3)-d-Gal, β-d-Galp-(1 → 4)-d-Gal, β-d-Galp-(1 → 6)-d-Gal, β-d-Galp-(1 → 3)-d-Lac, β-d-Galp-(1 → 4)-d-Lac, β-d-Galp-(1 → 6)-d-Lac were purchased from Carbosynth (Berkshire, UK).
3. Results
3.1. Expression of recombinant β-galactosidase from S. thermophilus
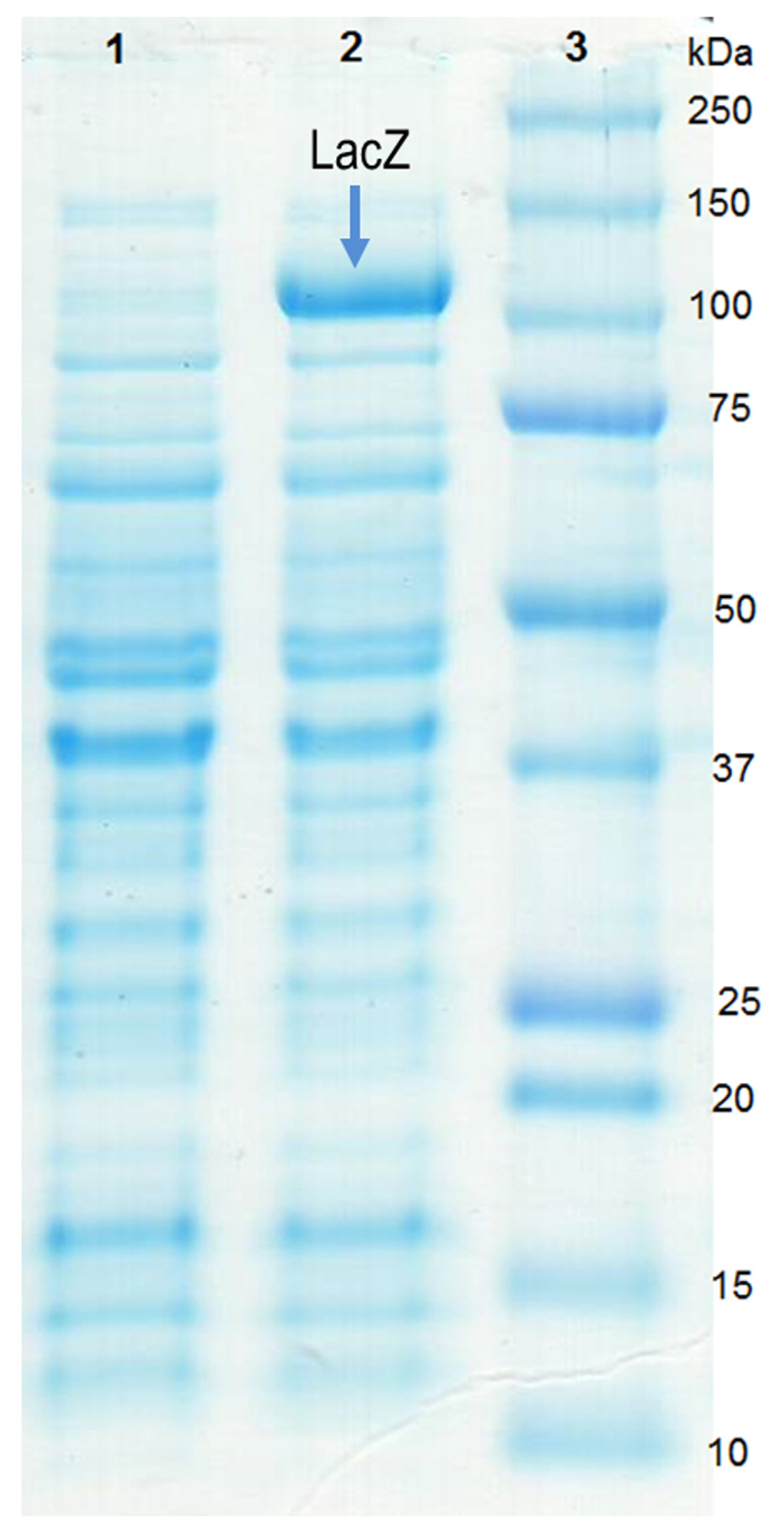
The yields of β-galactosidase activity when using the wild-type strain of S. thermophilus as a producer were rather low. For example, after cultivation of S. salivarius subsp. thermophilus DSM 20259 at 37°C for 24 h, the β-galactosidase yield, as measured in a cell-free extract, was only ~90 UoNPG per L of culture (M17 containing 2% lactose) with a specific activity of 2.1 U/mg (data not shown), which is activity of enzyme per mg protein. Hence, we set out to establish heterologous overexpression in a food-grade organism. To do so, we cloned the S. thermophilus lacZ gene into the pSIP409 vector for subsequent overexpression in L. plantarum. Induced and non-induced cells carrying the expression plasmid were harvested at OD600 of 1.8–2.0. SDS-PAGE analysis of cell-free protein extracts showed a unique band at ~100 kDa in induced L. plantarum cells (Fig. 1), which is in agreement with the calculated molecular mass of β-galactosidase from S. thermophilus.
Fig. 1.
SDS-PAGE of crude protein extracts obtained from noninduced (lane 1) and induced (lane 2) L. plantarum cells carrying the plasmid pSIP409lacZSt. The precision plus protein™ dual color standard (lane 3) was from Bio-Rad.
L. plantarum harboring p409lacZSt was then cultivated on a larger scale (1-L cultivation volume). Analysis of cell-free extracts of such 1-L laboratory cultivations showed enzyme yield of approximately 11 ± 0.5 kUoNPG of β-galactosidase activity. The specific activity of the extracts was ~12 U/mg. A small portion of the crude extract was used to purify the β-galactosidase to homogeneity by affinity chromatography using p-aminobenzyl 1-thio-β-d-galactopyranoside immobilized onto cross-linked 4% beaded agarose (Sigma) and the specific activity of the purified enzyme was determined to be 65 U/mg (data not shown), therefore the enzyme yield of 11 ± 0.5 kUoNPG of β-galactosidase activity per liter of culture corresponds to approximately 170 mg of enzyme. The β-galactosidase activity in L. plantarum cells without plasmid was negligible (0.07 U/mg), and hence the enzyme activities obtained can be attributed solely to plasmid-encoded LacZ from S. thermophilus.
The steady-state kinetic constants were determined for the hydrolysis of lactose and o-nitrophenyl-β-d-galactopyranoside (oNPG). Kinetic analysis with lactose as the substrate showed Michaelis-Menten kinetics with the following parameters obtained by nonlinear regression: vmax = 17.67 ± 0.36 (μmol d-glucose released min−1 (mg protein)−1) and Km = 5.12 ± 0.53 (mM). The experiments with oNPG showed substrate inhibition and the following kinetic parameters were obtained: vmax = 151 ± 10 (μmol oNP released min−1 (mg protein)−1), Km = 0.55 ± 0.10 (mM), and Ki,s = 14.1 ± 2.3 (mM). The kcat values were 34.4 ± 0.70 (s−1) and 294.7 ± 20.3 (s−1) and the catalytic efficiencies (kcat/Km) were 6.7 and 539 (mM−1 s−1) for lactose and oNPG, respectively.
3.2. Stability of the crude enzyme
Temperature stability of this crude enzyme preparation in sodium phosphate buffer (pH 6.5) and in whey permeate solution (dissolved in sodium phosphate buffer, pH 6.5) was measured at 37°C and 50°C (Table 1). The half-life time (t1/2) of the crude enzyme in whey permeate solution was increased compared to sodium phosphate buffer. A possible explanation could be the presence of the substrate and various mono- and divalent ions, such as sodium or magnesium, in the whey, which can affect the stability of β-galactosidases. The effect of ions such as Na+ on activity and Mg2+ on thermal stability seems common among GH2 β-galactosidases and is observed for E. coli β-galactosidase LacZ [31] as well as for some purified β-galactosidases from Lactobacillus spp. [27,32–34]. The effect of additional Mg2+ on stability of the crude enzyme in sodium phosphate buffer and in whey permeate was also tested and the results show that the addition of Mg2+ tends to have further stabilizing effect. Importantly, the data show that the recombinantly expressed enzyme is quite stable at 50°C.
Table 1.
Half-life times (t1/2) of activity (in) for crude recombinant β-galactosidase from S. thermophilus overexpressed in L. plantarum.
| Temperature (°C) | Sodium phosphate buffer (pH 6.5) | Sodium phosphate buffer (pH 6.5) + 10 mM MgCl2 | Whey permeate (dissolved in sodium phosphate buffer, pH 6.5) | Whey permeate (dissolved in sodium phosphate buffer, pH 6.5 + 10 mM MgCl2) |
|---|---|---|---|---|
| 37 | 25.4 | nd | 33.6 | 39.6 |
| 50 | 21.0 | 27.5 | 25.1 | 28.1 |
nd: not determined.
Experiments were performed in duplicates, and the standard deviation was always <5%.
3.3. Lactose hydrolysis and formation of GOS
Based on the observations of the stability of the crude enzyme preparation, the conversion of lactose in whey using the crude recombinant β-galactosidase from S. thermophilus was performed at both 37°C and 50°C to investigate the influence of process temperature. Whey permeate powder containing 65% (w/w) lactose was dissolved in 50 mM sodium phosphate buffer, pH 6.5 with 10 mM MgCl2 to a concentration of 50 g L−1 lactose, which is about the concentration of lactose in liquid whey and in milk. Crude enzyme was added to a final concentration of 5 UoNPG (corresponding to 1.35 ULac) per mL of reaction mixture. Lactose hydrolysis was significantly faster at 50°C and was completed within 2 h of reaction, while at 37°C lactose was completely hydrolyzed only after 5 h (Table 2). A maximum GOS yield of 34.2% of total sugar mass was achieved at ~80% lactose conversion at 37°C. At ~94–95% lactose conversion, the GOS yield decreased to ~30%, and when lactose was completely hydrolyzed, the GOS yield was ~20%. The reduction in GOS content can be explained by the fact that GOS are also subject to hydrolysis. When continuing the reaction after complete lactose conversion had been achieved, GOS concentrations decreased even further to ~11% after 7 h at 37°C or after 3 h at 50°C.
Table 2.
Lactose conversion and GOS formation during hydrolysis of lactose (50 g L−1) in whey permeate solution dissolved in 50 mM sodium phosphate buffer (pH 6.5) and 10 mM MgCl2, at 37 °C and 50 °C using 5 UoNPG/mL of crude recombinant β-galactosidase from S. thermophilus overexpressed in L. plantarum.
| Time (h) | 37 °C |
50 °C |
||
|---|---|---|---|---|
| GOS (% mass of total sugars) | Lactose conversion (%) | GOS (% mass of total sugars) | Lactose conversion (%) | |
| 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 32.2 | 70.3 | 29.1 | 95.3 |
| 2 | 34.2 | 79.7 | 16.8 | >99.8 |
| 3 | 31.2 | 93.7 | 11.1 | –– |
| 4 | 25.0 | 96.6 | ||
| 5 | 22.1 | >99.8 | ||
| 7 | 10.7 | –– | ||
3.4. GOS production
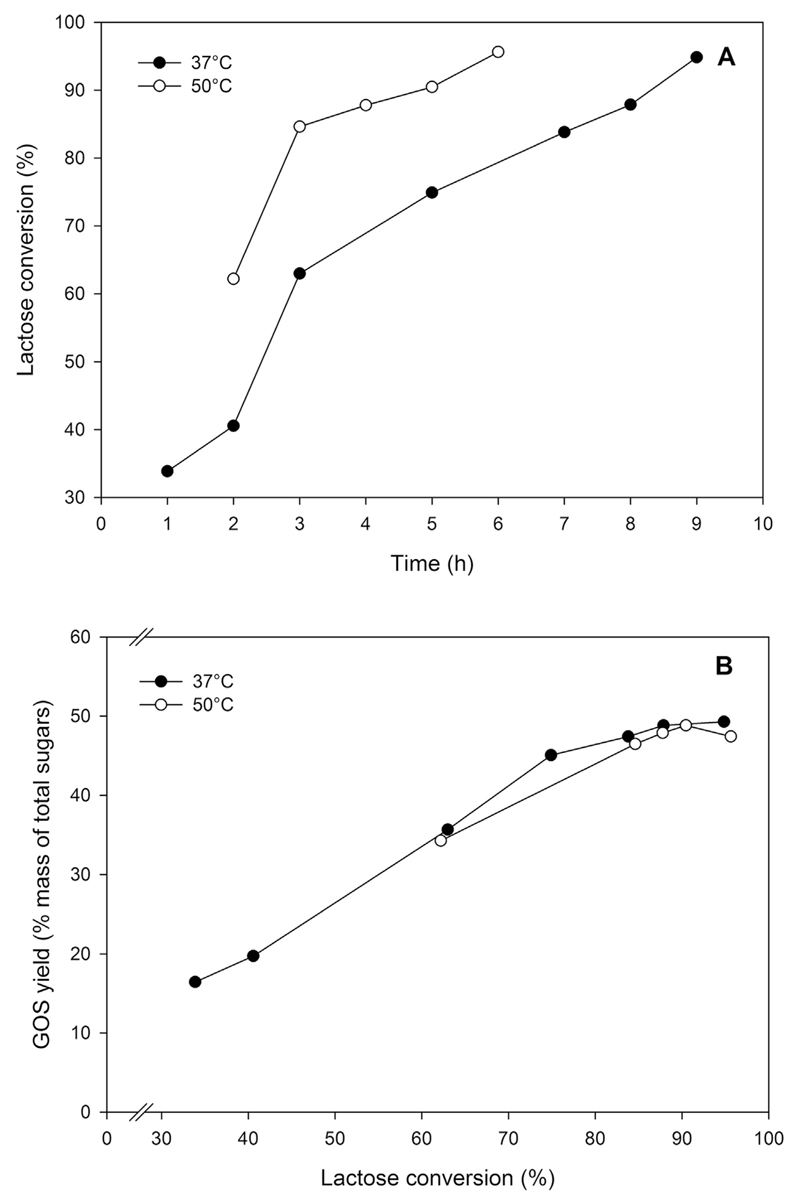
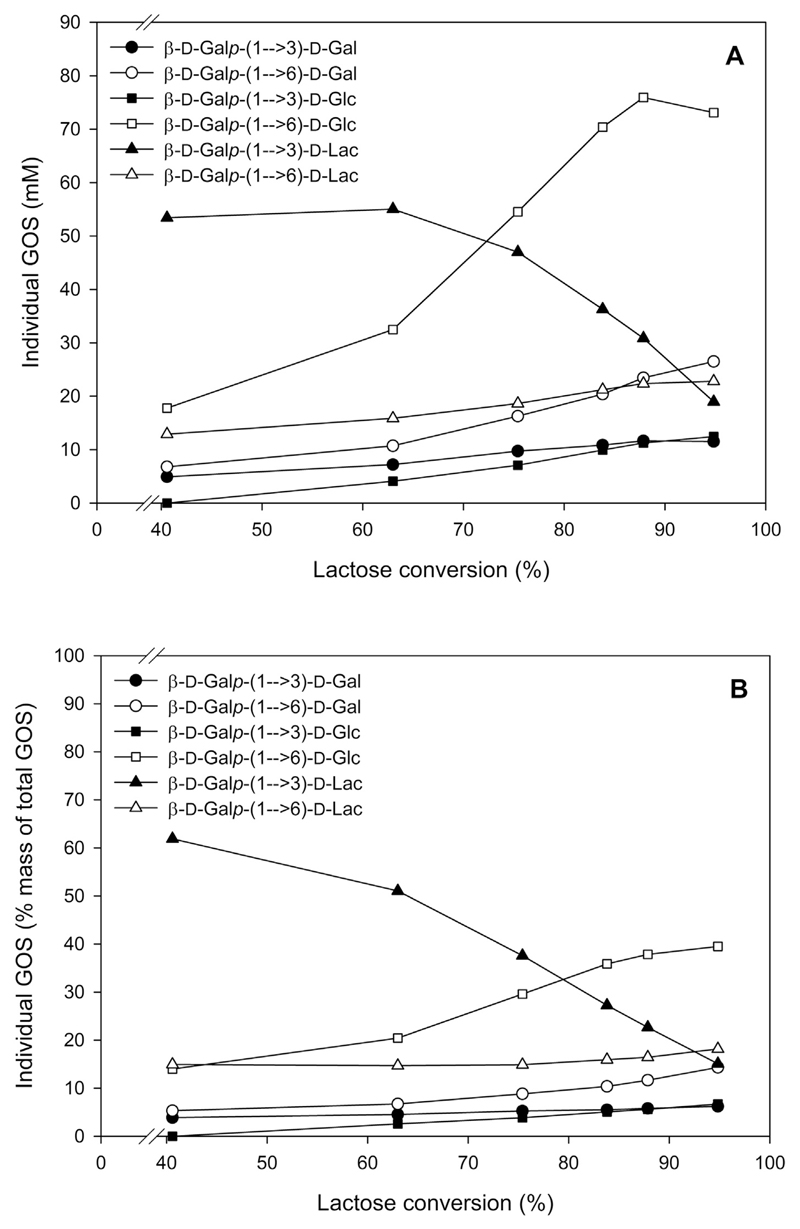
The formation of GOS described above happened even at low initial lactose concentration (50 g L−1). To increase the GOS yields further, we also tested a higher initial lactose concentration for this reaction. Whey permeate powder containing 65% (w/w) lactose was dissolved in 50 mM sodium phosphate buffer, pH 6.5 with 10 mM MgCl2 to an equivalent concentration of 205 g L−1 lactose. The reaction was again performed at 37°C and 50°C using 10 UoNPG/mL (corresponding to 2.7 ULac/mL) of enzyme. At 50°C, it took 5 h and 6 h to achieve ~90% and ~95% lactose conversion, respectively. The conversion was slower at 37°C, with ~95% lactose conversion being obtained after 9 h of reaction (Fig. 2A). The maximum GOS yield was ~50% of total sugar mass at ~90% lactose conversion, which was reached after 5 h of reaction at 50°C and 8.5 h of reaction at 37°C (Fig. 2B). Using authentic standards, we could identify the main GOS products of transgalactosylation, which are β-d-Galp-(1 → 6)-d-Glc, β-d-Galp-(1 → 3)-d-Lac, β-d-Galp-(1 → 3)-d-Glc, β-d-Galp-(1 → 3)-d-Gal, β-d-Galp-(1→ 6)-d-Gal, β-d-Galp-(1 → 6)-d-Lac (Fig. 3).
Fig. 2.
Time course of lactose conversion (A) and GOS yields (B) of reactions run at 37°C and 50°C using whey permeate dissolved in 50 mM sodium phosphate buffer, pH 6.5 with 10 mM MgCl2 to a final concentration corresponding to 205 g L−1 lactose. Reactions were performed using 10 UoNPG/mL of crude recombinant β-galactosidase from S. thermophilus overexpressed in L. plantarum.
Fig. 3.
Formation and degradation of individual GOS, in mM (A) and as mass percentage of total GOS (B) during lactose conversion at 37°C. Reactions were performed as described for Fig. 2.
4. Discussion
Lactic acid bacteria (LAB) are important microorganisms in the food and beverage industry. Over the past few decades, LAB have been used not only as starter cultures but also as producers of flavoring enzymes, antimicrobial peptides or metabolites that contribute to the flavor, texture and safety of food products [35–37]. LAB have for a long time been used in the production of a wide range of foods without adverse effects on humans. Due to their food-grade status and probiotic characteristics, several LAB are considered as safe and effective cell-factories for food-application purposes [36,37]. Due to this potential, several constitutive or inducible gene expression and protein targeting systems have been developed for LAB [23,35,36,38]. One such expression system comprises the so-called pSIP vectors [24] and is based on promoters and regulatory genes involved in the production of the class-II bacteriocins sakacin A [39] and sakacin P [40,41] in Lactobacillus spp. One of the advantages of this system is that it is strictly regulated and leads to high production of the target protein.
Recently, we reported the overproduction of β-galactosidases from Lactobacillus reuteri and Lactobacillus bulgaricus in the food-grade expression host L. plantarum WCSF1 [34,42]. The heterodimeric β-galactosidase of L. reuteri is encoded by two overlapping genes, lacL and lacM, while the homodimeric β-galactosidase of L. bulgaricus is encoded by lacZ. These enzymes both belong to glycoside hydrolase family GH2. The predominant GH2 β-galactosidases found in lactobacilli are of the LacLM type, encoded by the overlapping lacLM genes [22,32,33,43], while GH2 β-galactosidases of the LacZ type, encoded by a single lacZ gene, are less frequently found in lactobacilli. These LacZ β-galactosidases are more frequent in other LAB including S. salivarius and S. thermophilus [44] or bifidobacteria including Bifidobacterium bifidum [45], Bifidobacterium longum subsp. infantis [46], or Bifidobacterium breve [47]. When overexpressing β-galactosidases from L. reuteri and L. bulgaricus in the host L. plantarum WCFS1, the highest yields obtained under optimized fermentation conditions were ~35–40 kU and ~53 kU of β-galactosidase activity per liter of culture, respectively [34,48]. The yield obtained for β-galactosidase from S. thermophilus overexpressed in L. plantarum WCFS1 was somewhat lower, namely ~11 kU per liter of fermentation medium. Notably, this yield was obtained using relatively simple fermentation conditions without optimization and is expected to improve significantly if the fermentation process is optimized, as previously shown for the overexpression of β-galactosidase from L. reuteri in L. plantarum WCFS1 [48].
The present study was performed with crude recombinant β-galactosidase obtained after cell disruption and separation of cell debris by centrifugation. Because of the GRAS status of L. plantarum, it is safe to use a crude extract in food and feed applications. The direct use of a crude extract can reduce the enzyme costs of the process by avoiding laborious and expensive purification steps. An advantage of this recombinant β-galactosidase from S. thermophilus is that it is stable at high temperatures (up to 50°C) for an extended period of time. The enzyme is stable at 50°C with a t1/2 of more than a day, whereas the process for GOS production reported in this study can be completed within 5 h to obtain the maximal GOS yield. One major drawback of using mesophilic biocatalysts in industrial processes is the threat of microbial contamination. Most GH2 β-galactosidases from LAB are mesophilic biocatalysts, with some exceptions, for example β-galactosidase from L. bulgaricus [34] and the enzyme reported here in this study. Performing these conversion experiments at increased temperature will obviously decrease the chance of microbial contamination.
We first looked at lactose hydrolysis at a low initial lactose concentration of 50 g L−1, which is the concentration of lactose in liquid whey and in milk. This approach could reveal whether the enzyme has potential for removal of lactose in whey liquid waste or for applications in the dairy industry, such as production of low-lactose or lactose-free products or prevention of crystallization of lactose in dairy products. The recombinantly produced S. thermophilus β-galactosidase was found to be a promising candidate for these applications as complete lactose hydrolysis was obtained within less than 2 h at reasonable enzyme dosage. We did not detect lactose in our samples after 5 h at 37°C and 2 h at 50°C (Table 2) by using HPAEC-PAD for analysis, which confirms complete lactose hydrolysis. The detection limit of our HPAEC-PAD system for lactose is 0.1 g L−1. The level of lactose was also analyzed with the enzyme-based lactose biosensor Lactosens (DirectSens GmbH, Vienna, Austria; http://www.directsens.com), which confirmed the concentration to be below 0.1 g L−1, which is the limit recommended for dairy products to be labelled as ‘lactose-free’[49].
A more attractive biocatalytic application of the β-galactosidase is based on its transgalactosylation potential. Indeed, recombinant β-galactosidase from S. thermophilus was found to be suitable for the production of GOS, with the highest total GOS yields reaching ~50% when the enzyme was used in batch conversion mode with an initial lactose concentration of 205 g L−1. This yield can be considered as relatively high compared to the reported yields obtained with other β-galactosidases from LAB and some commercial β-galactosidases, for example L. reuteri (38%) [30], L. sakei (41%) [32], L. plantarum (41%) [33], Lactobacillus pentosus (31%) [43], L. bulgaricus (49.5%) [34], Kluyveromyces lactis (Lactozym 3000 L HP G from Novozymes, Bagsvaerd, Denmark) (30%) [50], Bacillus circulans (Biolacta FN5 from Daiwa Kasei K.K., Japan) (39%) [51]. GOS yields of over 50% are not often exceeded, and more typical optimized yields are between 30% and 40% (w/w) [52]. The increase in GOS yield is often observed with increased initial lactose concentration [52], therefore higher GOS yield than the yield reported here might be even achieved with higher initial lactose concentration. When looking at the individual components of the GOS mixture, it becomes evident that the recombinant enzyme from S. thermophilus has a propensity to synthesize β-(1 → 6) and β-(1 → 3)-linked GOS. Such a preference towards β-(1 → 3)- and β-(1 → 6)-bond formation has also been found for other β-galactosidases from LAB [30,32–34,43]. The predominant transgalactosylation products were identified as β-d-Galp-(1 → 6)-d-Glc (allolactose) and β-d-Galp-(1 → 3)-d-Lac (Fig. 3). It was reported that the administration of a GOS mixture containing β-(1 → 3) as well as β-(1 → 4) and β-(1 → 6) linkages proved to have a better bifidogenic effect than a mixture containing GOS with β-(1 → 4) and β-(1 → 6) linkages [53].
The use of whey permeate powder as a cheap lactose source could lower the costs for production of GOS. We have previously reported a process for GOS production using crude enzyme extract from Lactobacillus sp. [54]. In this previously reported process, we used the native enzyme, whose production yield was in the order of 2.5 kU of β-galactosidase activity per liter of culture [27]. The reaction temperature was as low as 17°C to limit possible microbial contamination, and therefore the obtained GOS yield from whey permeate was only ~25% after 15 h [54]. The process described in the present study has significant improvements, which are: (1) higher production of recombinant β-galactosidase in a food-grade organism, which lowers the enzyme costs, (2) the use of a thermostable crude recombinant enzyme, which enables the process to be carried out at 50°C, reducing the risks of microbial contamination, and (3) high GOS yield amounting up to ~50% of total sugar, which can be obtained in a much shorter process time, that is within 5 h at 50°C.
5. Conclusion
We describe the valorization of whey using an enzymatic approach for the production of GOS. Overexpression of β-galactosidase from S. thermophilus in another food-grade organism as well as the direct use of a crude cell extract reduce the enzyme costs. The process is very efficient with 50% of GOS yield being obtained within 5 h at 50°C. Our results imply that 1 kg of GOS can be produced from 2 kg of lactose or 3 kg or whey permeate powder using ~100 kU of enzyme. The conversion of lactose in whey into valuable products such as GOS would substantially decrease the environmental impact of whey, while significant profits could be obtained.
Acknowledgement
THN and RK acknowledge the support from the Austrian Science Fund (FWF Projects P24868-B22, P25313-B20). HMN acknowledges the COST Action TD1203 for STSM grant for her research visit to the Protein Engineering and Proteomics Group at NMBU, Ås, Norway, and also thanks VIED (Vietnam International Education Development) and OeAD for financial support. HMN and BG are grateful for support from the doctoral program BioToP—Biomolecular Technology of Proteins (grant FWF-W1224 of the Austrian Science Fund). This work is the result of the collaboration between the Food Biotechnology Laboratory at BOKU—University of Natural Resources and Life Sciences, Vienna, and the Protein Engineering and Proteomics Group at NMBU, the Norwegian University of Life Sciences, under the COST Action TD1203 (EUBis).
References
- [1].Smithers GW. Whey and whey proteins—from ‘gutter-to-gold’. Int Dairy J. 2008;18:695–704. [Google Scholar]
- [2].Mandal S, Puniya M, Sangu KPS, Dagar SS, Singh R, Puniya AK. Dairy by-products, wastes, or resources: the shifting perception after valorization. In: Chandrasekaran M, editor. Valorization of Food Processing By-products. CRC Press Taylor & Francis Group; 2012. pp. 617–648. [Google Scholar]
- [3].Guimarães PMR, Teixeira JA, Domingues L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv. 2010;28:375–384. doi: 10.1016/j.biotechadv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [4].Bozanic R, Barukcic I, Lisak Jakopovic K, Tratnik L. Possibilities of whey utilisation, Austrin. J Nutr Food Sci. 2014;2:7. [Google Scholar]
- [5].Banaszewska A, Cruijssen F, Claassen GDH, van der Vorst JGAJ. Effect and key factors of byproducts valorization: the case of dairy industry. J Dairy Sci. 2014;97:1893–1908. doi: 10.3168/jds.2013-7283. [DOI] [PubMed] [Google Scholar]
- [6].Nakayama T, Amachi T. β-Galactosidase, enzymology. In: Flickinger MC, Drew SW, editors. Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation. John Willey and Sons; New York: 1999. pp. 1291–1305. [Google Scholar]
- [7].Pivarnik LF, Senecal AG, Rand AG. Hydrolytic and transgalactosylic activities of commercial beta-galactosidase (lactase) in food processing. In: Kinsella JE, Taylor SL, editors. Advances in Food and Nutrition Research. Academic Press Inc; 1995. pp. 1–102. [DOI] [PubMed] [Google Scholar]
- [8].Prenosil JE, Stuker E, Bourne JR. Formation of oligosaccharides during enzymatic lactose hydrolysis: part I: state of art. Biotechnol Bioeng. 1987;30:1019–1025. doi: 10.1002/bit.260300904. [DOI] [PubMed] [Google Scholar]
- [9].Petzelbauer I, Zeleny R, Reiter A, Kulbe KD, Nidetzky B. Development of an ultra-high-temperature process for the enzymatic hydrolysis of lactose: II. Oligosaccharide formation by two thermostable β-glycosidases. Biotechnol Bioeng. 2000;69:140–149. doi: 10.1002/(sici)1097-0290(20000720)69:2<140::aid-bit3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [10].Holzapfel WH, Schillinger U. Introduction to pre- and probiotics. Food Res Int. 2002;35:109–116. [Google Scholar]
- [11].Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS Microbiol Ecol. 2005;52:145–152. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [12].Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- [13].Broekaert WF, Courtin CM, Verbeke K, van de Wiele T, Verstraete W, Delcour JA. Prebiotic and other health-related effects of cereal-derived arabinoxylans arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- [14].Crittenden RG, Playne MJ. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol. 1996;7:353–361. [Google Scholar]
- [15].De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res. 2011;55:46–57. doi: 10.1002/mnfr.201000451. [DOI] [PubMed] [Google Scholar]
- [16].Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–1188. doi: 10.1111/j.1399-3038.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- [17].Qiang X, YongLie C, QianBing W. Health benefit application of functional oligosaccharides. Carbohydr Polym. 2009;77:435–441. [Google Scholar]
- [18].Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- [19].Gopal PK, Sullivan PA, Smart JB. Utilisation of galacto-oligosaccharides as selective substrates for growth by lactic acid bateria including Bifidobacterium lactis DR10 and Lactobacillus rhamnous DR20. Int Dairy J. 2001;11:19–25. [Google Scholar]
- [20].Sangwan V, Tomar SK, Singh RRB, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- [21].Kleerebezem M, Boekhorst J, Van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWEJ, Stiekema W, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nguyen TH, Splechtna B, Yamabhai M, Haltrich D, Peterbauer C. Cloning and expression of the β-galactosidase genes from Lactobacillus reuteri in Escherichia coli. J Biotechnol. 2007;129:581–591. doi: 10.1016/j.jbiotec.2007.01.034. [DOI] [PubMed] [Google Scholar]
- [23].Sørvig E, Mathiesen G, Naterstad K, Eijsink VGH, Axelsson L. High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology. 2005;151:2439–2449. doi: 10.1099/mic.0.28084-0. [DOI] [PubMed] [Google Scholar]
- [24].Sørvig E, Grönqvist S, Naterstad K, Mathiesen G, Eijsink VGH, Axelsson L. Construction of vectors for inducible gene expression in Lactobacillus sakei and L. plantarum. FEMS Microbiol Lett. 2003;229:119–126. doi: 10.1016/S0378-1097(03)00798-5. [DOI] [PubMed] [Google Scholar]
- [25].Aukrust T, Blom H. Transformation of Lactobacillus strains used in meat and vegetable fermentations. Food Res Int. 1992;25:253–261. [Google Scholar]
- [26].Eijsink VGH, Brurberg MB, Middelhoven PH, Nes IF. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J Bacteriol. 1996;178:2232–2237. doi: 10.1128/jb.178.8.2232-2237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nguyen T-H, Splechtna B, Steinböck M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D. Purification and characterization of two novel β-galactosidases from Lactobacillus reuteri . J Agric Food Chem. 2006;54:4989–4998. doi: 10.1021/jf053126u. [DOI] [PubMed] [Google Scholar]
- [28].Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF. Stability of biocatalysts. Curr Opin Chem Biol. 2007;11:220–225. doi: 10.1016/j.cbpa.2007.01.685. [DOI] [PubMed] [Google Scholar]
- [29].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [30].Splechtna B, Nguyen TH, Steinböck M, Kulbe KD, Lorenz W, Haltrich D. Production of prebiotic galacto-oligosaccharides from lactose using β-galactosidases from Lactobacillus reuteri. J Agric Food Chem. 2006;54:4999–5006. doi: 10.1021/jf053127m. [DOI] [PubMed] [Google Scholar]
- [31].Juers DH, Rob B, Dugdale ML, Rahimzadeh N, Giang C, Lee M, Matthews BW, Huber RE. Direct and indirect roles of His-418 in metal binding and in the activity of β-galactosidase (E. coli) Protein Sci. 2009;18:1281–1292. doi: 10.1002/pro.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Iqbal S, Nguyen TH, Nguyen HA, Nguyen TT, Maischberger T, Kittl R, Haltrich D. Characterization of a heterodimeric GH2 β-galactosidase from Lactobacillus sakei Lb790 and formation of prebiotic galacto-oligosaccharides. J Agric Food Chem. 2011;59:3803–3811. doi: 10.1021/jf103832q. [DOI] [PubMed] [Google Scholar]
- [33].Iqbal S, Nguyen TH, Nguyen TT, Maischberger T, Haltrich D. β-galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr Res. 2010;345:1408–1416. doi: 10.1016/j.carres.2010.03.028. [DOI] [PubMed] [Google Scholar]
- [34].Nguyen TT, Nguyen HA, Arreola SL, Mlynek G, Djinović-Carugo K, Mathiesen G, Nguyen TH, Haltrich D. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and biochemical characterization. J Agric Food Chem. 2012;60:1713–1721. doi: 10.1021/jf203909e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Vos WM. Safe and sustainable systems for food-grade fermentations by genetically modified lactic acid bacteria. Int Dairy J. 1999;9:3–10. [Google Scholar]
- [36].De Vos WM. Gene expression systems for lactic acid bacteria. Curr Opin Microbiol. 1999;2:289–295. doi: 10.1016/S1369-5274(99)80050-2. [DOI] [PubMed] [Google Scholar]
- [37].Kuipers OP, De Ruyter PGGA, Kleerebezem M, De Vos WM. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 1997;15:135–140. doi: 10.1016/S0167-7799(97)01029-9. [DOI] [PubMed] [Google Scholar]
- [38].Diep DB, Mathiesen G, Eijsink VG, Nes IF. Use of lactobacilli and their pheromone-based regulatory mechanism in gene expression and drug delivery. Curr Pharm Biotechnol. 2009;10:62–73. doi: 10.2174/138920109787048571. [DOI] [PubMed] [Google Scholar]
- [39].Axelsson L, Holck A. The genes involved in production of and immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Bacteriol. 1995;177:2125–2137. doi: 10.1128/jb.177.8.2125-2137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brurberg MB, Nes IF, Eijsink VG. Pheromone-induced production of antimicrobial peptides in Lactobacillus. Mol Microbiol. 1997;26:347–360. doi: 10.1046/j.1365-2958.1997.5821951.x. [DOI] [PubMed] [Google Scholar]
- [41].Huhne K, Axelsson L, Holck A, Krockel L. Analysis of the sakacin P gene cluster from Lactobacillus sake Lb674 and its expression in sakacin-negative Lb. sake strains. Microbiology. 1996;142(Pt 6):1437–1448. doi: 10.1099/13500872-142-6-1437. [DOI] [PubMed] [Google Scholar]
- [42].Halbmayr E, Mathiesen G, Nguyen TH, Maischberger T, Peterbauer CK, Eijsink VGH, Haltrich D. High-level expression of recombinant β-galactosidases in Lactobacillus plantarum and Lactobacillus sakei using a sakacin p-based expression system. J Agric Food Chem. 2008;56:4710–4719. doi: 10.1021/jf073260+. [DOI] [PubMed] [Google Scholar]
- [43].Maischberger T, Leitner E, Nitisinprasert S, Juajun O, Yamabhai M, Nguyen TH, Haltrich D. β-galactosidase from Lactobacillus pentosus: Purification, characterization and formation of galacto-oligosaccharides. Biotechnol J. 2010;5:838–847. doi: 10.1002/biot.201000126. [DOI] [PubMed] [Google Scholar]
- [44].Vaillancourt K, Moineau S, Frenette M, Lessard C, Vadeboncoeur C. Galactose and lactose genes from the galactose-positive bacterium Streptococcus salivarius and the phylogenetically related galactose-negative bacterium Streptococcus thermophilus: Organization, sequence, transcription, and activity of the gal gene products. J Bacteriol. 2002;184:785–793. doi: 10.1128/JB.184.3.785-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goulas TK, Goulas AK, Tzortzis G, Gibson GR. Molecular cloning and comparative analysis of four β-galactosidase genes from Bifidobacterium bifidum NCIMB41171. App Microbiol Biotechnol. 2007;76:1365–1372. doi: 10.1007/s00253-007-1099-1. [DOI] [PubMed] [Google Scholar]
- [46].Hung MN, Xia Z, Hu NT, Lee BH. Molecular and biochemical analysis of two β-galactosidases from Bifidobacterium infantis HL96. Appl Environ Microbiol. 2001;67:4256–4263. doi: 10.1128/AEM.67.9.4256-4263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Arreola SL, Intanon M, Suljic J, Kittl R, Pham NH, Kosma P, Haltrich D, Nguyen TH. Two β-galactosidases from the human isolate Bifidobacterium breve DSM 20213: Molecular cloning and expression, biochemical characterization and synthesis of galacto-oligosaccharides. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nguyen TT, Nguyen HM, Geiger B, Mathiesen G, Eijsink VGH, Peterbauer CK, Haltrich D, Nguyen TH. Heterologous expression of a recombinant lactobacillal β-galactosidase in Lactobacillus plantarum: effect of different parameters on the sakacin P-based expression system. Microb Cell Fact. 2015:1–11. doi: 10.1186/s12934-015-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Erich S, Kuschel B, Schwarz T, Ewert J, Böhmer N, Niehaus F, Eck J, Lutz-Wahl S, Stressler T, Fischer L. Novel high-performance metagenome β-galactosidases for lactose hydrolysis in the dairy industry. J Biotechnol. 2015;210:27–37. doi: 10.1016/j.jbiotec.2015.06.411. [DOI] [PubMed] [Google Scholar]
- [50].Martínez-Villaluenga C, Cardelle-Cobas A, Corzo N, Olano A, Villamiel M. Optimization of conditions for galactooligosaccharide synthesis during lactose hydrolysis by β-galactosidase from Kluyveromyces lactis (Lactozym 3000 L HP G) Food Chem. 2008;107:258–264. [Google Scholar]
- [51].Palai T, Mitra S, Bhattacharya PK. Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade β-galactosidase. J Biosci Bioeng. 2012;114:418–423. doi: 10.1016/j.jbiosc.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [52].Gosling A, Stevens GW, Barber AR, Kentish SE, Gras SL. Recent advances refining galactooligosaccharide production from lactose. Food Chem. 2010;121:307–318. [Google Scholar]
- [53].Depeint F, Tzortzis G, Vulevic J, I’Anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008;87:785–791. doi: 10.1093/ajcn/87.3.785. [DOI] [PubMed] [Google Scholar]
- [54].Splechtna B, Nguyen TH, Zehetner R, Lettner HP, Lorenz W, Haltrich D. Process development for the production of prebiotic galacto-oligosaccharides from lactose using β-galactosidase from Lactobacillus sp. Biotechnol J. 2007;2:480–485. doi: 10.1002/biot.200600230. [DOI] [PubMed] [Google Scholar]