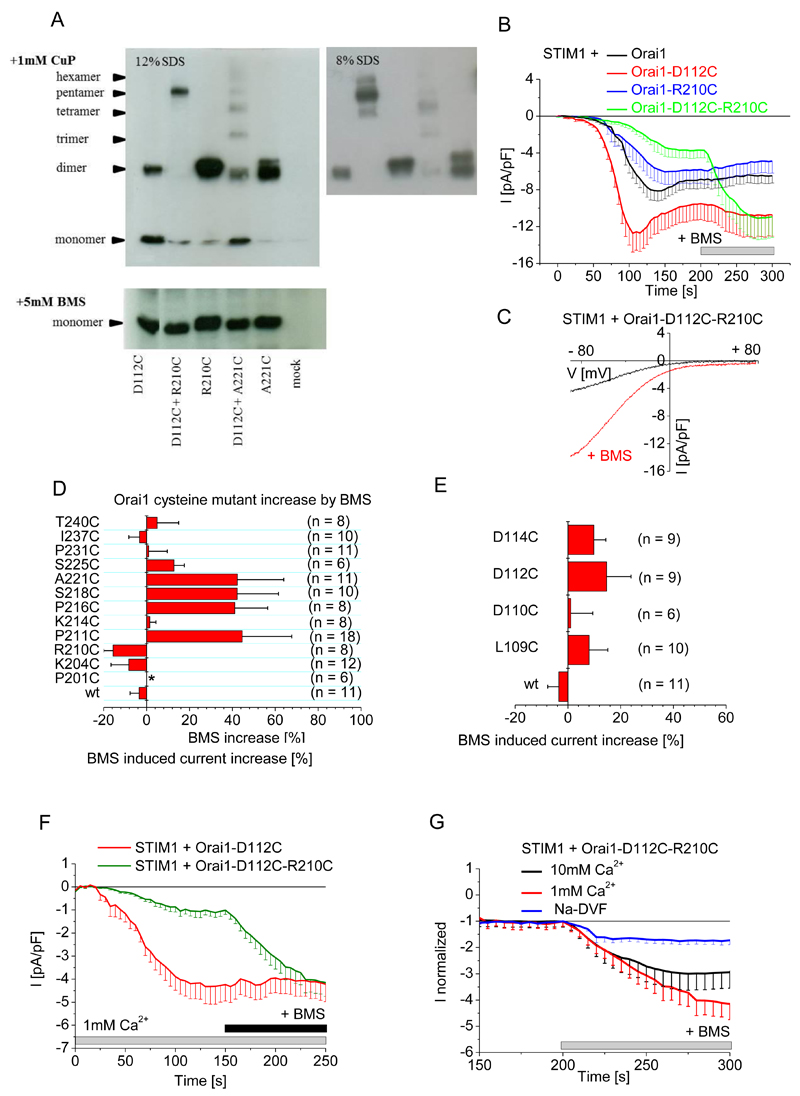
Figure 6. Disruption of CAR through forced dimerization between loop1 and loop3 inhibits SOCE.
(A) Oligomerization of the indicated Orai1 single and double cysteine mutants overexpressed in HEK 293 cells were detected by SDS PAGE (12%, left; 8% right) after incubation with 1mM CuP (upper panel) or after the addition of 5 mM BMS (lower panel). (B) Whole-cell patch-clamp experiments show time course of currents mediated by Cherry-tagged STIM1 coexpressed with wild-type eYFP-tagged Orai1 or Orai1 mutant as indicated in HEK cells exposed to 20 mM EGTA in the patch pipette and a 10 mM Ca2+-containing solution, and upon maximum current activation, BMS (5 mM) was added (n = 8 - 11). Analysis by t-test, with p < 0.05 for statistical significance, indicated that Orai1-D112C maximum currents are significantly different from those of Orai1-D112C-R210C in the absence but not in the presence of BMS. (C) Current-voltage relationship of the store-operated activation of Orai1-D112C-R210C and upon BMS stimulation from a representative experiment from (B). (D) The BMS-dependent increase in currents mediated by wild-type (wt) eYFP-tagged Orai1 and the indicated eYFP-tagged loop3 cysteine mutants upon maximum store-dependent activation. All Orai1 mutants, except P201C, yielded a store-dependent current when coexpressed with STIM1 in HEK 293 cells exposed to 20 mM EGTA in the patch pipette and a 10 mM Ca2+-containing solution. Data are shown as the mean + SEM. (E) The BMS-dependent increase in currents mediated by wild-type (wt) eYFP-tagged Orai1 and the indicated YFP-tagged loop1 cysteine mutants upon maximum store-dependent activation under the same conditions as in (D). (F) Whole-cell patch-clamp experiments show time course of currents mediated by Cherry-tagged STIM1 coexpressed with wild-type eYFP-tagged Orai1 or Orai1 mutant in a 1mM Ca2+ containing bath solution (n= 7-11 cells). Maximum currents of Orai1-D112C and Orai1-D112C-R210C were analyzed by t-test before and after addition of BMS for statistical significance, and are significantly different (p < 0.05) before addition of BMS. (G) Comparison of relative BMS-dependent stimulation in currents mediated by Cherry-tagged STIM1 and eYFP-tagged Orai1-D112C-R210C coexpressed in HEK 293 cells in the presence of the indicated extracellular solutions (n = 6 - 11). Data were analyzed by t-test for statistical significance (p < 0.05) of maximum currents upon BMS treatment, determining that 1mM and 10mM Ca2+ currents are significantly different compared to sodium based currents.