Fig. 2.
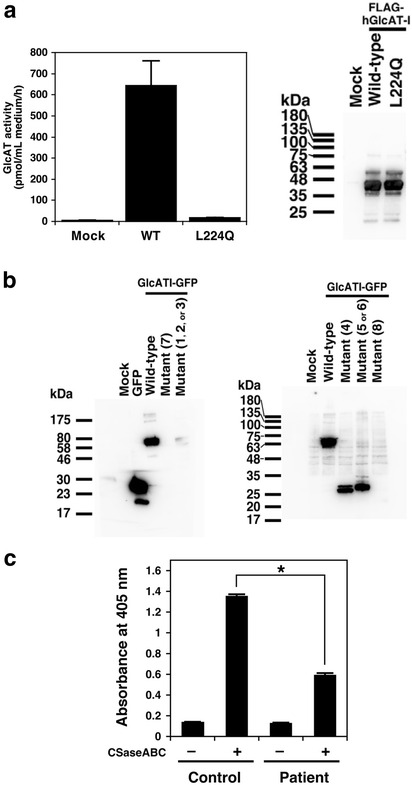
a Glucuronyltransferase activity of each enzyme protein was examined using the partially purified recombinant enzymes, UDP-[14C]-GlcA as the donor substrate, and Galβ1-3Galβ1-O-methyl as the acceptor substrate. Glucuronyltransferase activity was determined by incorporation of [14C]GlcA into the Galβ1-3Galβ1-O-methyl. “Mock” indicates the enzyme source from the HEK293T cells transfected with an empty vector. Values are the means ± SE (n = 3). *P < 0.0005 versus wild-type was calculated by the ANOVA Dunnett test. Two sets of the mutant enzyme, L224Q, were prepared from two separate conditioned media from the HEK293T cell cultures (left). The purified recombinant GlcAT-I separated by SDS-PAGE was detected with the horseradish peroxidase-conjugated anti-FLAG antibody (Wako). The broad signals of the FLAG-tagged GlcAT-I may indicate the N-glycosylated enzymes (~40 kDa). Western blotting analysis showed the expression of comparable amounts of these recombinant proteins. (right). b The GFP-tagged GlcAT-I and its mutant proteins separated by SDS-PAGE were detected with anti-GFP antibody and the horseradish peroxidase-conjugated anti-mouse IgG antibody as the primary and secondary antibodies, respectively. The deduced molecular masses of GFP-tagged wild-type GlcAT-I and the mutant proteins (mutants #1 ~ 8) were summarized in Additional file 1: Table S2. Although no obvious mutant proteins (No. 1 ~ 3, 7, and 8) of GlcAT-I were detected, GFP-tagged mutant proteins, #4 as well as #5 or 6, were clearly detected. Predicted molecular weights of the bands of #5 and #6 were 40.2 and 31.3 kDa, respectively (Additional file 1: Table S2). The GFP-tagged mutant protein #5 or 6 was detected at a molecular weight of ~ 30 kDa, suggesting that the observed GFP-tagged mutant protein might be mutant-#6 rather than mutant-#5. c Comparison of the amount of CS chains on the cell surface of fibroblasts from the patient and a healthy control by cell-based ELISA. *P < 0.0001
