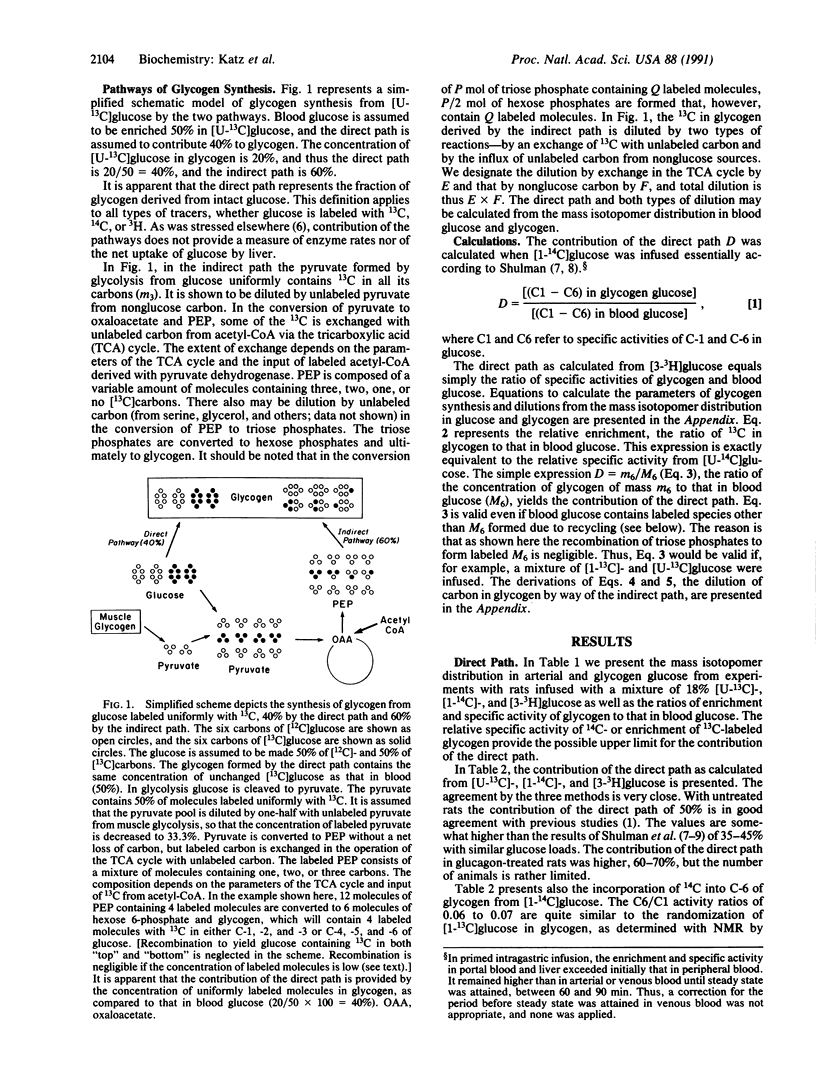
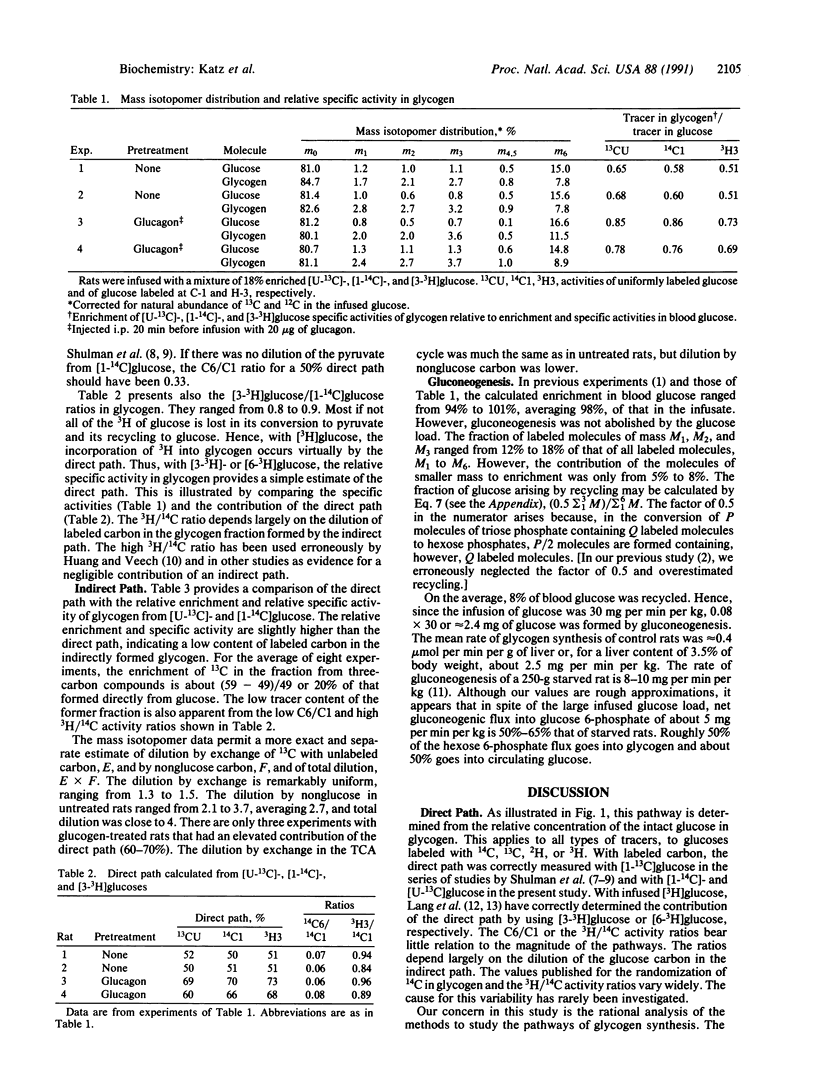
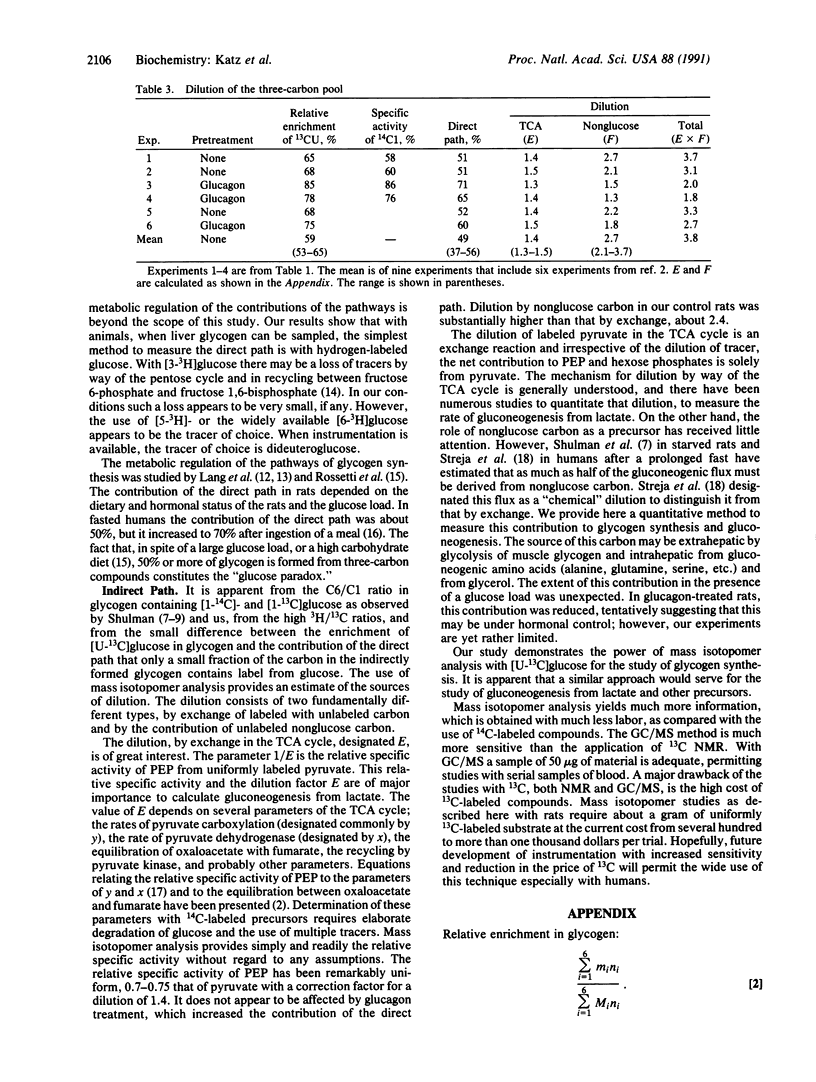
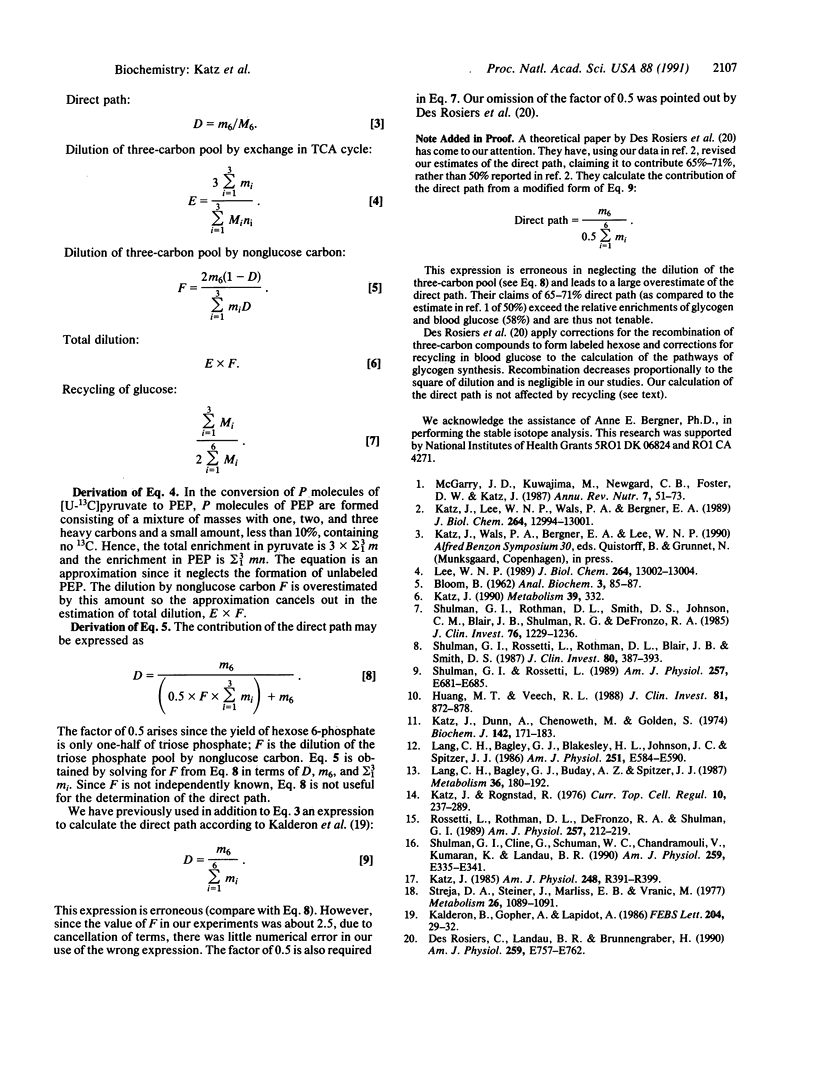
Abstract
Rats were infused with glucose at 30 mg/min, containing 18% enriched [U-13C]glucose and [1-14C]- and [3-3H]glucose. The mass isotopomer patterns of 13C-labeled blood glucose and liver glycogen were determined by gas chromatography/mass spectroscopy. The contribution of the direct pathway to glycogen was calculated from the three tracers, and the values by all three were nearly identical, about 50%. The 14C specific activity in carbon 6 of glycogen glucose was about 6% that of carbon 1. The [3H]glucose/[1-14C]glucose ratio in glycogen was 80-90% that in blood glucose. The enrichment of 13C and the specific activity of 14C in glycogen formed by the indirect path were 20-25% of glycogen formed directly from glucose. The dilution is of two kinds: (i) an exchange of labeled carbon with unlabeled carbon in the tricarboxylic acid cycle and (ii) dilution by unlabeled nonglucose carbon. Methods to calculate the two types of dilution are presented. In control rats the dilution factor by exchange in the tricarboxylic acid cycle is 1.4, and the dilution by unlabeled carbon is 2.5- to 3.0-fold, with the overall dilution about 4-fold. In rats preinjected with glucagon, the dilution through the tricarboxylic acid cycle was unaffected but that by nonglucose carbon was decreased.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Des Rosiers C., Landau B. R., Brunengraber H. Interpretation of isotopomer patterns in tracing glycogen synthesis and glucose recycling using [13C6]glucose. Am J Physiol. 1990 Nov;259(5 Pt 1):E757–E762. doi: 10.1152/ajpendo.1990.259.5.E757. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Veech R. L. Role of the direct and indirect pathways for glycogen synthesis in rat liver in the postprandial state. J Clin Invest. 1988 Mar;81(3):872–878. doi: 10.1172/JCI113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon B., Gopher A., Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett. 1986 Aug 11;204(1):29–32. doi: 10.1016/0014-5793(86)81381-3. [DOI] [PubMed] [Google Scholar]
- Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol. 1985 Apr;248(4 Pt 2):R391–R399. doi: 10.1152/ajpregu.1985.248.4.R391. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Lee W. N., Wals P. A., Bergner E. A. Studies of glycogen synthesis and the Krebs cycle by mass isotopomer analysis with [U-13C]glucose in rats. J Biol Chem. 1989 Aug 5;264(22):12994–13004. [PubMed] [Google Scholar]
- Katz J. On the measurement of pathways of glycogen synthesis. Metabolism. 1990 Mar;39(3):331–332. doi: 10.1016/0026-0495(90)90058-k. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Blakesley H. L., Johnson J. L., Spitzer J. J. Plasma glucose concentration determines direct versus indirect liver glycogen synthesis. Am J Physiol. 1986 Nov;251(5 Pt 1):E584–E590. doi: 10.1152/ajpendo.1986.251.5.E584. [DOI] [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Buday A. Z., Spitzer J. J. The contribution of gluconeogenesis to glycogen repletion during glucose infusion in endotoxemia. Metabolism. 1987 Feb;36(2):180–187. doi: 10.1016/0026-0495(87)90015-1. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Cline G., Schumann W. C., Chandramouli V., Kumaran K., Landau B. R. Quantitative comparison of pathways of hepatic glycogen repletion in fed and fasted humans. Am J Physiol. 1990 Sep;259(3 Pt 1):E335–E341. doi: 10.1152/ajpendo.1990.259.3.E335. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rossetti L. Influence of the route of glucose administration on hepatic glycogen repletion. Am J Physiol. 1989 Nov;257(5 Pt 1):E681–E685. doi: 10.1152/ajpendo.1989.257.5.E681. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rossetti L., Rothman D. L., Blair J. B., Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987 Aug;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Smith D., Johnson C. M., Blair J. B., Shulman R. G., DeFronzo R. A. Mechanism of liver glycogen repletion in vivo by nuclear magnetic resonance spectroscopy. J Clin Invest. 1985 Sep;76(3):1229–1236. doi: 10.1172/JCI112078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streja D. A., Steiner G., Marliss E. B., Vranic M. Turnover and recycling of glucose in man during prolonged fasting. Metabolism. 1977 Oct;26(10):1089–1098. doi: 10.1016/0026-0495(77)90035-x. [DOI] [PubMed] [Google Scholar]


