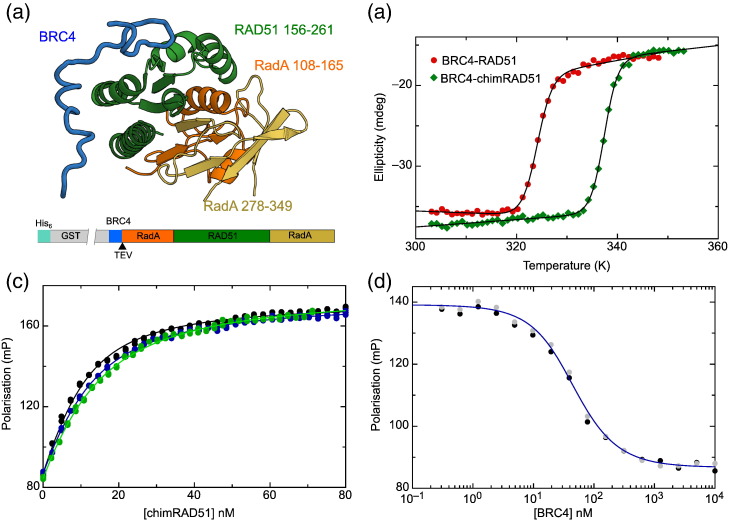
Fig. 4.
Design and validation of the chimeric RAD51 protein. (a) Domain structure of RAD51:BRC4 complex highlighting the parts that make up the chimeric protein. RadA N- and C-terminal parts are coloured orange and beige, respectively, with the central part of RAD51 in green. The diagram below shows the complete expression construct with His6-GST fusion, BRC4 protein with TEV cleavage site, and the ChimRAD51. (b) Thermal denaturation curves for BRC4–RAD51 (green) and BRC4–ChimRAD51 (red) following CD signal at 208 nm. (c) FP binding assay between ChimRAD51 and the fluorescently labelled BRC4 peptide. Each of the three curves and associated measurements represents an independent experiment. (d) Competition FP measurement using unlabelled BRC4 peptide to complete the Alexa Fluor 488-labelled peptide. Data are shown for two independent titrations, and the blue line is fitted to the average.