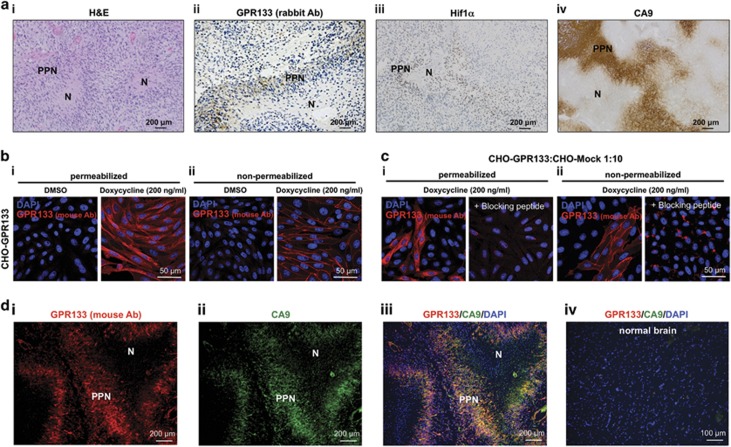
Figure 2.
Expression of GPR133 overlaps with hypoxia markers in GBM biospecimens. (ai) Hematoxylin and eosin (H&E) of a human GBM showing the necrotic (N) and psesudopalisading necrosis (PPN) regions within the tumor. (ii–iv) Representative images of immunohistochemistry in human GBM biospecimens. A rabbit polyclonal anti-GPR133 antibody reveals selective expression of GPR133 in PPN (ii), along with hypoxic markers Hif1α (iii) and CA9 (iv). (b) Validation of mouse monoclonal GPR133 antibody using CHO cells inducibly overexpressing GPR133. (i) Permeabilized cells showed immunoreactivity at the cell membrane and intracellularly, likely reflecting trafficking of GPR133 along the secretory pathway. (ii) Immunoreactivity was confined to the cell membrane in non-permeabilized cells. In both (i) and (ii), GPR133 immunoreactivity was detected only after induction with doxycycline (200 ng/ml). (c) GPR133 immunoreactivity was abolished by the blocking peptide. (d, i–iii) Immunofluorescent analysis of GPR133 and CA9 in biospecimens. Costaining using a mouse monoclonal antibody against GPR133 in formalin-fixed paraffin-embedded (FFPE) biospecimens also confirms that GPR133 expression highly overlaps with CA9 in (7/8 biospecimens, one biospecimen failed to show PPN). (iv) Normal brain shows neither CA9 nor GPR133 immunoreactivity (using the mouse monoclonal antibody). Ab, antibody.