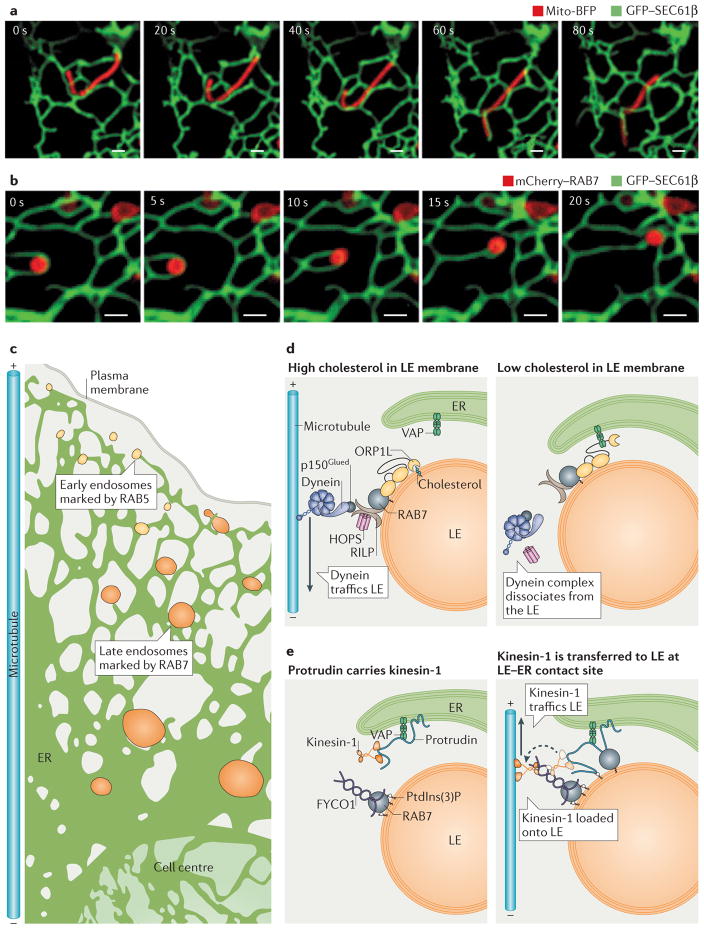
Figure 2. Dynamics of endoplasmic reticulum (ER) membrane contact sites (MCSs).
a,b | Endosomes and mitochondria are tightly tethered to ER tubules even as they traffic. Time-lapse fluorescence images of ER and organelle dynamics in live Cos-7 cells expressing GFP–SEC61β (labelling ER in green) and (a) mito-BFP (labelling mitochondria in red) or (b) mCherry–RAB7 (labelling late endosomes (LEs) in red). Note how the contact sites are maintained as the apposing organelles move. Scale bars represent 1 μm. c | Endosomes mature as they traffic from the cell periphery along microtubules to the cell centre. ER–endosome contact increases as endosomes mature, with 53% of early endosomes (EEs; marked by RAB5) and 99% of LEs (marked by RAB7) remaining in contact with the ER during trafficking. d | Model of how cholesterol levels regulate the composition of ER–LEs MCSs and LE trafficking. When the LE contains high cholesterol levels (left panel), oxysterol-binding-related protein 1L (ORP1L) can bind to cholesterol on the LE membrane and does not associate with ER VAPs (VAMP-associated proteins). In addition, ORP1L interacts with RAB7 GTPase, which stimulates minus-end-directed LE trafficking through a complex that includes RILP (RAB-interacting lysosomal protein), the HOPS (homotypic fusion and vacuole protein sorting) complex, dynactin (p150Glued) and the motor protein dynein. At low cholesterol levels (right panel), ORP1L is not bound to cholesterol and instead interacts with ER VAPs. The ORP1L–VAP interaction displaces dynein from the endosome. e | Protrudin is an ER integral membrane protein that interacts with VAP and kinesin-1 (left panel). Protrudin binds to RAB7 and phosphatidylinositol-3-phosphate (PtdIns(3)P) on the LE membrane. Protrudin can bind to and transfer kinesin-1 to the LE protein FYCO1 (FYVE and coiled-coil domain-containing protein 1), and this promotes plus-end-directed microtubule trafficking of LEs (right panel).