Table 1. EC50, pEC50, and Efficacy Values for 1 and the Top Six HssRS Activators from the Single Point Screena.
| Cmpd | Structure | EC50(μM) | pEC50 | Efficacy (%) |
|---|---|---|---|---|
| 1 |
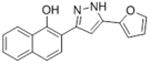
|
11.6 | 4.90 ± 0.37 | 100 |
| la |
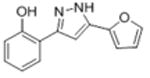
|
30.4 | 4.49 ± 0.083 | 28.2 ± 5.4 |
| lb |
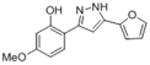
|
50.2 | 4.30 ± 0.20 | 26.2 ± 6.7 |
| 2a |
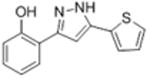
|
ND | ND | 17.0 ± 2.9 |
| 2c |
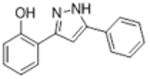
|
14.6 | 4.66 ± 0.016 | 87.8 ± 23.7 |
| 2d |
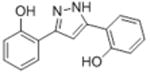
|
5.81 | 5.24 ± 0.076 | ND |
| 2e |
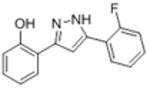
|
10.7 | 4.97 ± 0.033 | 35.7 ± 10.1 |
| 2f |
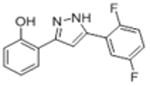
|
13.6 | 4.90 ± 1.1 | 30.1 ± 8.0 |
EC50 and pEC50 were calculated from concentration response curves after 6 h of growth using the XylE reporter assay.
An EC50 for 2a could not be determined because the concentration response curve did not plateau below its solubility limit. Efficacy is the percent activation of HssRS compared to 1 at 50 μM (all compounds reach Emax at or below 50 μM except 2a). All data were collected in triplicate, and error values are one standard deviation from the mean.
