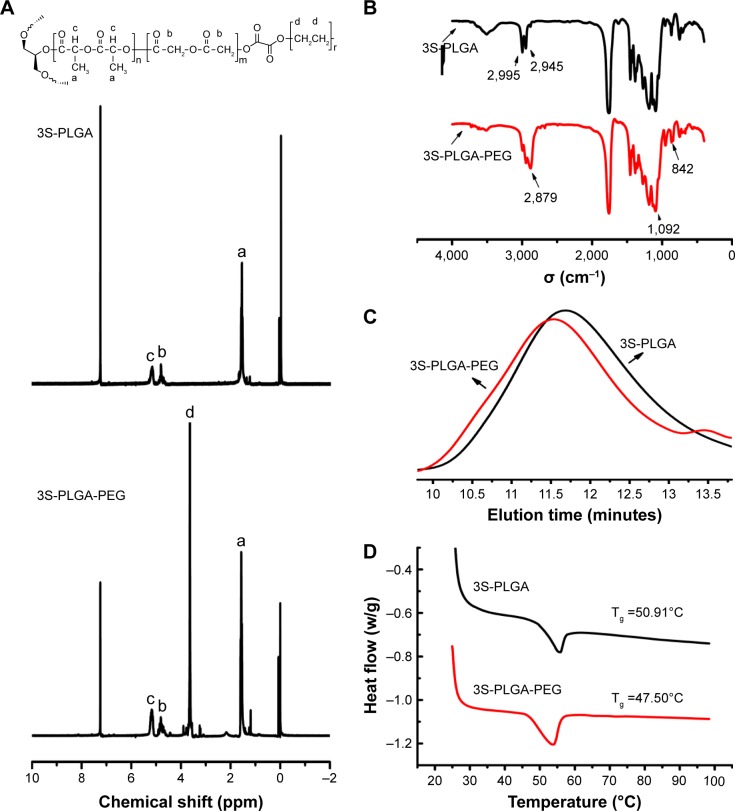
Figure 3.
(A) The chemical structure of 3S-PLGA-PEG was investigated by 1H-NMR spectra. 3S-PLGA was mainly determined by the appearance of the peaks of a (δ=1.55 ppm, –CH3 in PLGA segments), b (δ=4.82 ppm, –CH2 in PLGA segments), and c (δ=5.12 ppm, –CH in PLGA segments). Compared with the 3S-PLGA spectrum, the high intensity of peak d (δ=3.62 ppm, –CH2 in PEG segments) in the 3S-PLGA-PEG spectrum indicated the existence of PEG, which proved that 3S-PLGA-PEG was successfully synthesized. (B) FT-IR spectrometry: the characteristic peak of 3S-PLGA at 1,750 cm−1 was attributable to the C=O functional group, while the peaks at 2,995 cm−1 and 2,945 cm−1 were due to the stretching vibration of a saturated –CH bond. In the spectrum of 3S-PLGA-PEG, the emerging peaks of 2,879 cm−1, 1,092 cm−1, and 842 cm−1 proved the existence of the PEG chain. (C) Molecular weights and polydispersity were investigated by GPC: the peak of 3S-PLGA-PEG appeared earlier than the peak of 3S-PLGA, which proved that PEG was favorably linked with 3S-PLGA. (D) The glass transition temperature (Tg) of 3S-PLGA was about 50.91°C, while 3S-PLGA-PEG was 47.5°C. The existence of PEG affected the crystalline property of PLGA, which reduced the Tg of this polymer.
Abbreviations: 3S-PLGA, three-arm star block poly(lactic-co-glycolic acid); PEG, polyethylene glycol; NMR, nuclear magnetic resonance; FT-IR, Fourier-transform infrared; GPC, gel-permeation chromatography.