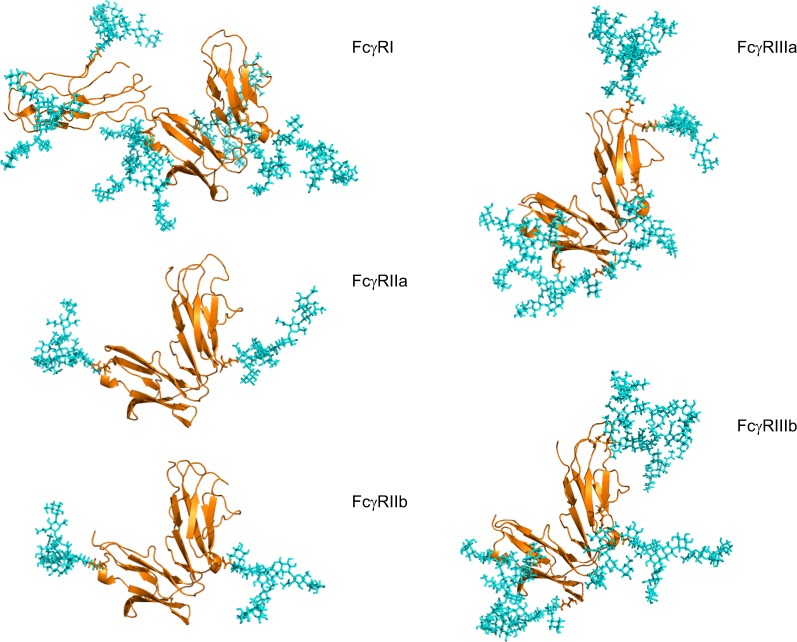
Figure 2.
Human FcγRs are complex glycoproteins.
Notes: FcγR ectodomains were modeled with N-glycans on each glycosylation site for each receptor, taking into account glycan size and composition, torsion angles and free energies. The N-glycans (shown in cyan) were modeled based on identified glycans from FcγRs (shown in gold) produced in recombinant systems such as NS0, CHO and HEK293 systems.43,47,50 In the case of FcγRIIIa, site-specific glycosylation studies were performed by Zeck et al,47 and this information was used to build the glycans shown on the Asn 162 site located at the binding interface with IgG. For the remaining receptors, no site-specific analysis was available, and in these cases the most abundant glycans identified from the recombinant sources were used to model N-glycosylation for these receptors. FcγRI has an extra D3 domain, which contributes to its high-affinity nature, and this domain also contains two glycosylation sites. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the Oxford notation (https://glycobase.nibrt.ie/glycobase/show_nibrt.action)86 and are as follows: FcγRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcγRIIa: Asn 64 (FA2G2S1), Asn 145 (FA2BG2). FcγRIIb: Asn 66 (FA2G2S1), Asn 147 (FA2BG2). FcγRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). FcγRIIIb: Asn 35 (FA2GalNAc2S2), Asn 42 (Man 5), Asn 61 (FA2G2S1), Asn 71 (FA3G2), Asn 159 (FA2BG1), Asn 166 (FA2BG2). PDB accession numbers used to build the models were as follows: FcγRI: 4×4m, FcγRIIa: 1fcg, FcγRIIb: 2fcb, FcγRIIIa: 3ay4, FcγRIIIb: 1e4j.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G; PDB, Protein Data Bank.