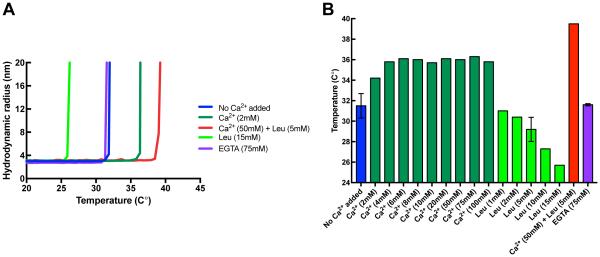
Fig. 2. Thermal stability of human mini-calpain-5.
(A) Representative thermal denaturation curves of human mini-calpain-5 wild type at 0.42 mg/ml with no Ca2+ added (blue) or on addition of 72 EGTA (purple), 10 mM Ca2+ (dark green), 50 mM Ca2+ with 5 mM leupeptin (red) or 5 mM leupeptin (light green) as determined by an increase in hydrodynamic radius on unfolding during a 1 °C/min temperature ramp by dynamic light scattering. (B) Concentration dependence of additives on mini-calpain-5 thermostability. Mini-calpain-5 starts unfolding at 32.0± 0.3°C, has a relatively unaltered Tonset of 31.6 ± 0.1 °C on addition of 75 mM EGTA, increasingly becomes more stable in the presence of Ca2+ (Tonset = 36.8 ± 0.1 °C; p ≤ 0.01) and is significantly more stable at 50 mM Ca2+ with 5 mM leupeptin (Tonset = 39.5 ± 0 °C; p ≤ 0.001). Increasing amounts of leupeptin alone, however, are destabilizing (Tonset = 25.7 °C at 15 mM leupeptin).