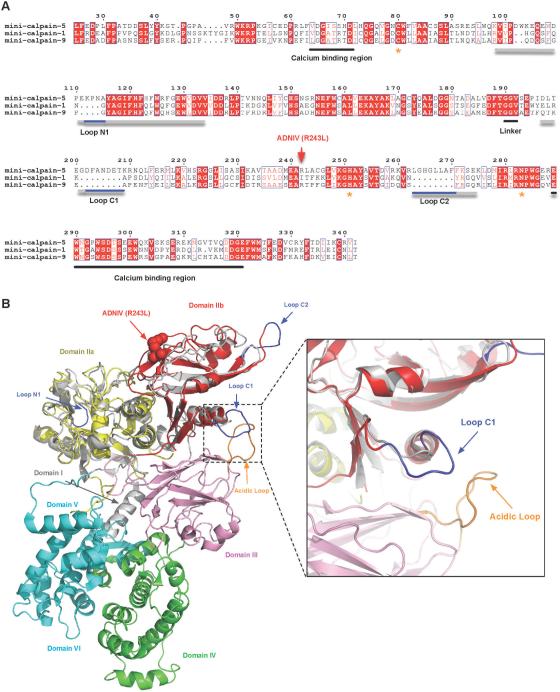
Fig. 5. Identification of loop extensions unique to calpain-5.
(A) Multiple sequence alignment of mini-calpain-1, -5, and -9. Loops N1, C1, and C2 are underlined in gray and with calpain-5 extensions, which appear as gaps in the mini-calpain-1 and -9 sequences, underlined in blue. Asterisks denote the catalytic residues. The calcium binding regions and the interdomain linker are underlined in black. The residue numbering shown is for calpain-5. (B) DIIa-IIb of a mini-calpain-5 homology model superimposed on the crystal structure of human calpain-2 (PDB ID: 1KFU). DIIa and DIIb of calpain-5 are colored yellow and red, respectively, while the corresponding calpain-1 domains are represented in gray. Extensions of the calpain-5 loops are colored in blue and correspond to the primary sequences shown in Fig. 5A. Loop C1 falls in close proximity to the calcium-binding acidic loop of DIII (inset).