Abstract
Objective
Ripples (80–150 Hz) recorded from clinical macroelectrodes have been shown to be an accurate biomarker of epileptogenic brain tissue. We investigated coupling between epileptiform spike phase and ripple amplitude to better understand the mechanisms that generate this type of pathological ripple (pRipple) event.
Methods
We quantified phase amplitude coupling (PAC) between epileptiform EEG spike phase and ripple amplitude recorded from intracranial depth macroelectrodes during episodes of sleep in 12 patients with mesial temporal lobe epilepsy. PAC was determined by 1) a phasor transform that corresponds to the strength and rate of ripples coupled with spikes, and a 2) ripple-triggered average to measure the strength, morphology, and spectral frequency of the modulating and modulated signals. Coupling strength was evaluated in relation to recording sites within and outside the seizure onset zone (SOZ).
Results
Both the phasor transform and ripple-triggered averaging methods showed ripple amplitude was often robustly coupled with epileptiform EEG spike phase. Coupling was more regularly found inside than outside the SOZ, and coupling strength correlated with the likelihood a macroelectrode’s location was within the SOZ (p<0.01). The ratio of the rate of ripples coupled with EEG spikes inside the SOZ to rates of coupled ripples in non-SOZ was greater than the ratio of rates of ripples on spikes detected irrespective of coupling (p<0.05). Coupling strength correlated with an increase in mean normalized ripple amplitude (p<0.01), and a decrease in mean ripple spectral frequency (p<0.05).
Significance
Generation of low-frequency (80–150 Hz) pRipples in the SOZ involves coupling between epileptiform spike phase and ripple amplitude. The changes in excitability reflected as epileptiform spikes may also cause clusters of pathologically interconnected bursting neurons to grow and synchronize into aberrantly large neuronal assemblies.
Introduction
Mesial temporal lobe epilepsy (MTLE) is the most common adult form of medically refractory epilepsy and is often amenable to resective epilepsy surgery1. When the seizure onset zone (SOZ) is unilateral and localized to the mesial temporal lobe, anteromesial temporal lobectomy is the recommended procedure, and up to 85% of patients are seizure-free after surgery2,3.
Differentiating MTLE patients with a primarily unilateral SOZ from patients with seizure onset zones that are bilateral independent, or also include neocortical regions, would be aided by a more precise inter-ictal neurophysiological biomarker of epileptogenic mesial temporal lobe brain tissue4. High frequency oscillations (HFOs) are brief (15–200 msec) bursts of neurophysiological activity that range in frequency between 80 and 600 Hz. They are considered a candidate biomarker of epileptogenic tissue in intracranial recordings5. The epileptogenic zone is not always congruent with the SOZ, and it may not be fully characterized due to spatial or limited duration of recordings by the intracranial electrodes during the epilepsy monitoring unit stay6.
Epileptogenic hippocampus and entorhinal cortex can be identified on the basis of increased interictal high frequency (fast) ripple (200–600 Hz) burst rates in experimental microelectrode recordings, but not usually HFO bursts of lower frequency ripples (80–200 Hz)7–9. High frequency, or fast ripples are believed to reflect summated action potentials from clusters of synchronously bursting pathologically-interconnected neurons10, while sharp wave-ripples in normal hippocampus, are considered important for memory encoding, consolidation, and recall11–14, and reflect summated inhibitory postsynaptic potentials15,16. However, in the epileptic brain, some ripple frequency oscillations are pathological, and clinical macroelectrode recordings show that increased rates of both interictal ripples and fast ripples delineate the SOZ17,18.
Epileptiform spikes are usually distinct in morphology, higher in amplitude, and shorter in duration, compared to physiological sharp waves19. Thus, ripples that occur during epileptiform spikes are likely to be pathological, and not normal phenomenon, i.e. pathological ripples (pRipples). The underlying mechanisms that generate low-frequency (80–150 Hz) pRipple on spikes have not yet been fully elucidated. We hypothesize that these pRipples may be generated when the changes in excitability that are reflected as epileptiform spikes, also cause growth and synchronization of clusters of pathologically interconnected bursting neurons in epileptogenic regions. Synchronizability of the neuronal assembly20 should manifest as coupling between the epileptiform spike phase and the ripple amplitude21,22, since the changes in excitability are related to the spike phase.
In the current study, we quantified coupling between epileptiform spike phase and ripple amplitude in the inter-ictal depth electrode EEG recordings from presurgical patients with drug-resistant seizures, and asked if this form of phase amplitude coupling was associated with the SOZ. We also determined whether the strength of this coupling influenced ripple amplitude and frequency. We performed computer-aided visual inspection of the iEEG, a novel phasor transform methodology, and ripple-triggered averaging measures that derive the waveform and strength of the modulatory signal.
Methods
1. Patient selection
The 12 patients involved in this retrospective study underwent depth electrode evaluation at the UCLA Seizure Disorder Center between 2010 and 2016, and were selected on the basis of 1) availability of inter-ictal recordings with a 2 kHz sampling rate, and 2) a mesial temporal lobe SOZ, confirmed by bilateral depth macroelectrode recordings.
2. Localization of electrode sites and 3D reconstructions
All patients were scanned pre-implant using a 3T MRI scanner, (Magnetom, Siemens Trio, Erlangen, Germany), with a protocol consisting of at least a T1-weighted MPRAGE sequence. For some patient post-resection scans were also performed. Post-implant computerized tomograms (CTs) contained signal artifact corresponding to the contacts of all implanted depth electrodes. CT images were non-linearly and manually coregistered to the pre-implant and post-resection T1-weighted images using FLIRT (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). Each individual intracranial macroelectrode contact was manually delineated using the AFNI program (https://afni.nimh.nih.gov/afni/). Freesurfer software (http://freesurfer.net/) was used to segment brain structures and cortical surfaces, and the 3D images were displayed using Slicer (https://www.slicer.org/). To differentiate macroelectrode contacts residing in gray or white matter, the pre-implant T1 weighted MRI was anatomically segmented into 94 gray and white matter regions of interest using BrainSuite (http://brainsuite.org) and verified via visual inspection.
3. Recording methods and selection of data
Clinical intracranial EEG (iEEG, 0.1–600 Hz; 2000 samples/sec; reference scalp Fz) was recorded from 7-contact depth electrodes (Ad-Tech Medical Instrument Corp. Racine WI) using a Stellate (XLTEK, San Diego, CA, U.S.A) or a Nihon-Kohden 128-channel NK 1200 long-term monitoring system (Nihon-Kohden America, Foothill Ranch, CA, U.S.A.). The recordings were acquired during a 35 to 60 minute epoch of mixed-stage sleep. Sleep was confirmed by video-EEG inspection revealing K-complexes, spindles, slow waves, and a paucity of muscle artifact. We did not perform concurrent EOG and EMG recordings. Anti-seizure drug blood serum levels at the time of the recording were not available. The seizure onset zone was defined by a consensus decision made amongst a team of board certified epileptologists on the basis of visual inspection of the iEEG using the criteria of identifying the electrodes first exhibiting the ictal onset pattern23.
4. HFO detection and quantification
Muscle and electrode artifacts in iEEG recordings were reduced using a custom independent component analysis (ICA) based algorithm (Supplementary Methods, Figure S1). After applying this ICA-based method, ripples were detected in the referential montage iEEG recordings per macroelectrode contact by utilizing a Hilbert-detector methodology (Supplementary Methods, Figure S1).
5. Distinguishing ripples on spikes and reducing ringing artifact
To distinguish ripples that occur during epileptiform spikes from all other ripples, we calculated the derivative of the peri-ripple band pass filtered (4–30 Hz) iEEG, and applied a threshold of 2 uV/msec. Ripple events that exceeded this threshold were inspected and categorized as ripples on spikes (Figure S1).
Oscillatory events can arise due to Gibb’s phenomenon as a result of high-pass filtering sharp transients such as a epileptiform spike24. We developed a custom algorithm to exclude these events (Supplementary Methods, Figure S3). The performance of this method was confirmed with visual inspection.
5. Quantifying ripple detection accuracy
In a subset of the macroelectrode recordings, (N=4 patients) the detector results were used to annotate the iEEG recording, and were compared to the gold standard of visual inspection. The overall sensitivity of the detector for ripples events during epochs containing artifact was 96.7% +/− 2%, and the overall precision of the detector was 88.7 +/− 6.5%.
The ratio of the ripple rates detected from macroelectrodes positioned in gray matter relative to the ripple rates detected from macroelectrodes in white matter was 4.03 +/− 1.9 (n=7, paired student’s t-test p<0.05).
6. Utilization of ripple phasors
To examine and quantify coupling between spike phase and ripple amplitude, we transformed each ripple on spike event into a ripple phasor calculated on the basis of the instantaneous phase ϕ(t) of the low frequency iEEG recording and the corresponding instantaneous amplitude a(t) during the duration of the ripple event (Figure S2).
Each ripple phasor was calculated using eqn 1:
| Eqn. 1 |
where v is the vector strength of the phasor, theta its phase angle, and a(t) and phi(t) are the respective instantaneous ripple amplitude and low frequency iEEG phase during the ripple across its duration[t..T] (Figure S2).
Phase locking among the multiple HFO phasors [n..N] detected from individual macroelectrodes was performed using Rayleigh’s test for circular non-uniformity by calculating the mean vector strength (r) using eqn 2.
| Eqn. 2 |
and deriving the Rayleigh Z-statistic eqn 3.
| Eqn. 3 |
Rayleigh’s test for circular non-uniformity assumes a null hypothesis of uniformity (or bimodal opposing directions), and is based on determining the mean phase angle and angular spread across the individual phasors.
We calculated the phase locked ripple phasor rate on the basis of 1) identifying electrodes in which the ripple phasors for all the detected ripple events exhibited statistically significant phase locking (Rayleigh’s test for circular non-uniformity, p<0.05), and 2) tallying the number of ripple phasors that were within +/− 90 degrees of the mean phase angle of the total population of ripple phasors. By convention, 0° is the peak of an oscillation, and 180° the trough.
7. Ripple triggered averaging
To identify and characterize the low-frequency waveforms and oscillations that modulate ripple amplitude, we used a ripple-triggered coupling methodology25. Trials of unfiltered iEEG, one second in duration, aligned by the time of the maximum instantaneous amplitude of the ripple events at 0.5 seconds were summated to derive a modulatory signal. The statistical significance of the modulatory signal was derived by computing 300 surrogates using phase shuffling, and calculating the peak-peak amplitude of the randomized signals. The modulated signal was calculated by convolving each 1 second unfiltered iEEG trial, with a ripple occurring at ~0.5 seconds, with complex Morlet wavelets with a width of 7 cycles, and a standard deviation of 3 cycles using Fieldtrip (http://www.fieldtriptoolbox.org/). The time-frequency representations for each trial were then averaged and normalized (using a z-score) to account for (1/f) spectral power.
8. Statistics
The SOZ rate ratio for ripple events and phase locked ripple phasor events was calculated using eqn 4.
| Eqn 4 |
The correlation between ripple rates, Rayleigh score, and phase-locked ripple rates and the likelihood an electrode was positioned in the SOZ was calculated by 1) rank ordering the rates and scores within each subject, 2) assigning the rank ordered electrode a binary assignment based on its location, 3) summating these assigned values for the rank ordered channels across patients, and 4) performing a linear regression analysis between rank order and the resulting SOZ probability. Paired and unpaired student’s t-tests were two tailed, with a 0.05 significance level. The Kuiper’s test was performed using the circular statistic toolbox in Matlab (http://www.mathworks.com/matlabcentral/fileexchange/10676-circular-statistics-toolbox--directional-statistics-).
Results
1. Description of patients and recordings
Patients with suspected temporal lobe epilepsy during scalp EEG in the epilepsy monitoring unit were selected for depth electrode EEG monitoring due to 1) bilateral inter-ictal and ictal findings 2) a normal brain MRI or PET, or 3) concern for a possible lateral temporal SOZ (Table 1). Only 12 of these patients were included in this study due to the availability of 30 minutes of artifact free inter-ictal recordings sampled at 2 kHz during sleep. We analyzed recordings from between 2–9 macroelectrode contacts on 7–10 depth electrodes per patient (Table 1). We did not analyze recordings from every macroelectrode contact due to excessive artifact that interfered with HFO detection. Overall, across the 12 patients, recordings from 640 distinct macroelectrode contacts were analyzed. Of the 640 distinct macroelectrode recordings, 147 were located in the SOZ.
Table 1.
Patient characteristics.
| Patient Age Sex |
Sleep Stage |
Scalp EEG |
MRI | PET hypo- metabolic |
iEEG macro- electrode sites |
iEEG IED |
iEEG SOZ |
Surg- ery |
Path | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 34 M |
SWS Delta = 92% |
IEDs L(T1)>R( T2), SOZ L(T1,T3) |
Normal | bi- temporal |
RA1–7, RAH1–7, REC1–7, RPHG1–7, ROF1–7, LA1–7, LAH1–7, LEC1–7, LPHG1–7, LOF1–7 |
L=R MT/L T |
L MT>R MT/LT |
None | N/A | N/A |
| 2 54 F |
1,2 Delta = 89% |
IEDs R(T4), SOZ R(T2,T4) |
Normal | R. temporal |
RA1–6, RMH2–6, RSTa1–3 RSTp2–5 RSS1–9 RIPa1–7 RIPp1–9 LMH1–5 |
R LT | R MT/LT |
R. MT, LT resect -ion |
Gli- osis |
Engel I@4yr |
| 3 52 F |
1,2 SWS Delta = 95% |
IEDs L(T1), SOZ L(T1)>R( T2) |
Normal | Normal | RA1,2,4–7 RMH1–6 ROF1,5–7 LA1–6 LEC1–7 LMH1–6 LOF1–6 |
R>L MT, |
R>>L MT |
R. ATL |
FCD Ic |
Engel II@3yr |
| 4 53 M |
1,2 Delta = 85% |
IEDs L(T1), SOZ L(broad) |
L. PCA infarct |
L. PCA territory |
REC1–7 RPHG1–7 LA1–6 LEC1,2– 4–7 LFSG1–3 LOTa1,2 LTOp3–5 LSO2–3 |
L MT> LT |
L MT | L. ATL |
Gli- osis |
Deceased |
|
5 47 F |
A,1,2,S WS Delta = 85% |
IEDs L(T1)=R( T2), SOZ L(T1)>R( T2) |
R inf. frontal abnormality |
L>R temporal |
RAH1–7 REC1– 4,6–7 ROF1–7 RPAR1–7 RAC1–7 LAH1–7 LEC1–7 LOF1–7 LAC1–7 |
R>L MT |
R>L MT |
none | N/A | N/A |
| 6 42 F |
A,1,2 Delta = 85% |
IEDs R(T2)>L( T1), SOZ L(T3)>R( T2) |
T2/FLAIR hyper- intense R.temporal pole |
R temporal- pole |
RA2–7 REC1–7 RMH2–7 RPHG1–7 ROF1–7 LA2–7 LEC1– 3,5–7 LAH2–7 LPHG4–7 |
L>R MT |
R MT | R. ATL |
FCD IIb |
No seizures@ 6 months |
| 7 35 F |
1,2 Delta = 76% |
IEDs R(T4)>R( T2), SOZ R(T4) |
s/p RNS removal |
R temporal |
RA1–7 REC1–6 RMH2–6 ROF1–6 RAC1–7 SMA2–3 RMC1–7 LEC1–6 LMH3–5 |
R MT | R MT>LT |
R. ATL |
Gli- osis |
Seizures continue @ 6 months |
| 8 35 F |
1,2 Delta |
IEDs R(T4)>L( T3) SOZ, R(T2), R(T4), left broad PFA |
R>L Peri- ventricular heterotopia cingulate poly- microgyria, hypothalami c hamartoma |
L>R temporal, and peri- ventricular |
REC2–6 RMH1–7 ROF1–7 RTO1–7 LA1–7 LEC2–6 LMH2–7 LPHG1–7 LTO1- 3,5–7 |
R MT | R MT/LT , L LT, R O |
None | None | N/A |
| 9 30 M |
1,2 Delta = 80% |
IEDs L(T1)=R( T2) SOZ,R(T2 ) |
Normal | R lateral i.e. (neo) temporal |
RA1–7 REC1–7 RMH1–7 RPHG1–6 RSTG1–7 ROF1–7 RAC1–7 LEC1–7 LAH1–7 |
L>R MT |
R>L MT |
None | N/A | N/A |
| 10 27 M |
1,2 Delta = 89% |
IEDs L(T1)=R( T2) SOZ, R(T4)=L( T1) |
R parietal resected AVM, R MTS |
R mesial temporal |
RA1–6 REC1–6 RMH1–6 RPHG1–7 LEC1–6 LMH1–6 RPC1–7 RTO1–6 RO1–4 |
R>L MT |
R>L MT |
None | N/A | N/A |
| 11 30 F |
2, SWS Delta -- 82% |
IEDs, R(T2,T4) SOZ R(T2,T4) |
Right orbitofrontal volume loss |
Right orbito- frontal |
REC1–6 RAH1–7 RPHG1–7 RSTG1–7 RA1–6 ROF1–5,7 RAF1–5,7 LEC1–7 LMH1–6 |
R MT= R LT |
R MT/LT |
R ATL |
TBD | TBD |
| 12 21 F |
2, SWS Delta 83% |
IEDs, L(T1) SOZ L(F7,T1) |
Left anterior temporal encephalo- cele |
L. temporal |
RAH3–7 REC1–7 RPHG1,2, 5–7 ROF1–5 LA1–4,6–7 LEC1–7 LMH1–7 LPHG1–7 |
L>R MT |
L MT | L ATL |
TBD | TBD |
Abbreviations: A: awake, 1: stage 1 sleep, 2: stage 2 sleep, SWS: slow wave sleep, L: left, R: right, IEDs: inter-ictalepileptiform discharges, SOZ: seizure onset zone, AVM: arteriovenous malformation, MTS: mesial temporal lobe sclerosis, A: amygdala, AH: anterior hippocampus, MH: middle hippocampus, EC: entorhinal cortex, PHG: para-hippocampal gyrus, FSG: fusiform gyrus, STa: superior temporal (anterior), STp: superior temporal (posterior), SS: superior temporal sulcus, OF: orbitofrontal, AC: anterior cingulate, PC: posterior cingulate, IPa: inferior parietal (anterior), IPp: inferior parietal (posterior), PAR: parietal, OTa: occipital-temporal (anterior), TOP: temporal-occipital (posterior), SO: superior occipital, SMA: supplementary motor area, MC: middle occipital, TO: temporal occipital, O: occipital, RNS: repetitive nerve stimulator, FLAIR: fluid attenuated inversion recovery, PCA: posterior cerebral artery, MT: mesial temporal intracranial electrodes, LT: lateral (i.e. neocortical) temporal intracranial electrodes, O: occipital, ATL: anterior temporal lobectomy, FCD: focal cortical dysplasia, TBD: to be determined, N/A: not applicable.
Scalp EEG findings provide the laterality and relative frequency of IEDs and seizures, as well as the electrode (in parenthesis) where inter-ictal discharges or seizures are maximal or phase reverse. Intracranial findings provide the localization and relative frequency of inter-ictal discharges, and seizures.
Of the 12 patients, a diagnosis of unilateral mesial temporal SOZ was made in 3 patients, bilateral mesial temporal SOZ in 4 patients, mesial and lateral (i.e. neocortical) temporal SOZ in 4 patients, and mesial, lateral temporal, and extra-temporal SOZ in 1 patient. All three patients with unilateral MTLE were offered an anterior temporal lobectomy, but conclusive post-resection seizure outcome results are not yet available (Table-1). One patient had a mesial temporal and temporal neocortical SOZ and was seizure free after a mesial and neocortical resection, while another had independent SOZs in the right and left mesial temporal structures, but underwent a palliative right anterior temporal lobectomy with an Engel II outcome.
2. Ripple Amplitude is coupled with epileptiform spike phase
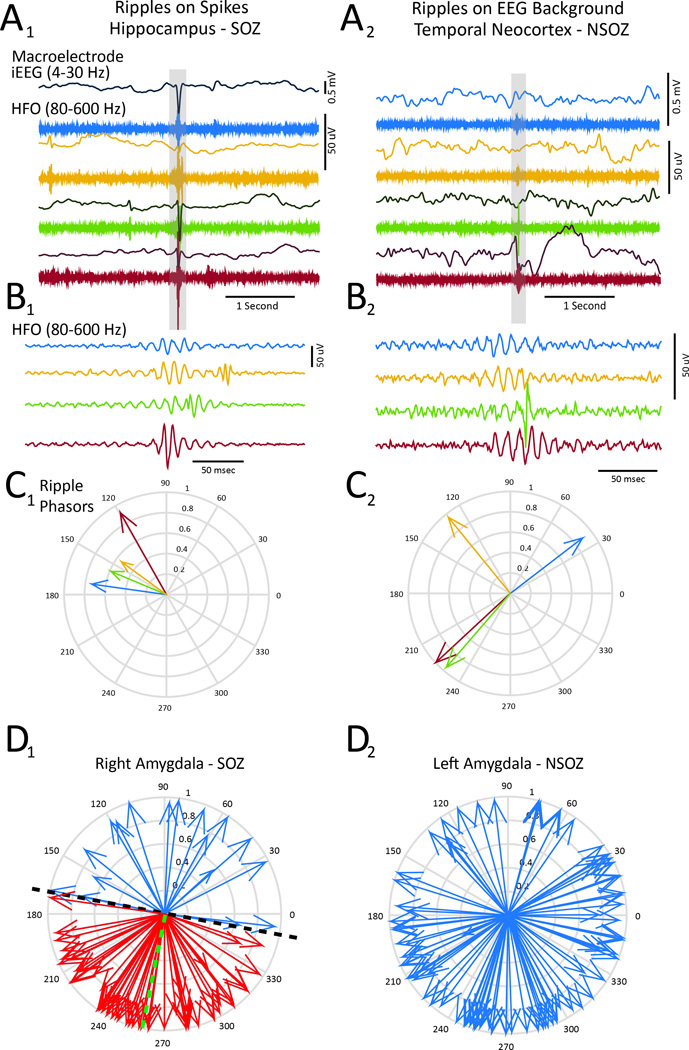
Visual inspection of the iEEG recordings demonstrated that ripples could occur with or without epileptiform EEG spikes (Figure 1A,B). Using the recordings from all 640 macroelectrodes, we detected a total of 28,957 ripples, and of these, 14,502 ripples were associated with an epileptiform spike. For each ripple, we calculated a “ripple phasor” (Figure S1, S2), a new measure which corresponds to the magnitude and phase angle between the instantaneous amplitude of the bandpass (80–150Hz) filtered ripple and the instantaneous phase of the Hilbert-transformed, bandpass (4–30Hz) filtered epileptiform spike or low frequency EEG in the absence of an epileptiform spike (Figures 1C1 & 1C2). In many cases, ripple phasors indicated that ripples occurred within a relatively narrow range of phase angles when associated with an epileptiform spike (Figure 1C1), compared to when ripples occurred in the absence of a spike (Figure 1C2). Subsequently, for each macroelectrode we determined if all the ripples on spikes (RonS) transformed to phasors were phase-locked (Figure-1D1, Rayleigh’s test for circular non-uniformity, p<0.05). If the ripple phasors exhibited phase locking, we tallied the number of ripple phasors with a respective phase angle within +/− 90° of the mean phase angle of the distribution (i.e. phase locked ripple phasors).
Figure 1.
Ripples can occur during epileptiform spikes or superimposed on the background EEG. (A) Four examples of the bandpass filtered (4–30 Hz, top) EEG and corresponding bandpass (80–600 Hz, middle) for ripples recorded from the hippocampus in the SOZ (left) and neocortical temporal contact (right) located contralateral to the SOZ. The traces are aligned to ripple events (gray bar). The ripples recorded from the hippocampus occurred during inter-ictal epileptiform EEG spikes, while neocortical ripples occurred during absence of EEG spikes. (B) Expansion of the aligned HFOs illustrated by the gray bar in A. (C) Polar plot of color-coded ripple phasors corresponding to ripples illustrated in panels A & B. Note the narrow range of phase angles associated with ripples associated with EEG spikes compared ripples that occurred in the absence of EEG spikes. (D) Polar plots of the ripple phasors (blue) and phase-locked ripple phasors (red) for events recorded from the right amygdala in the SOZ (D1), green dashed line represents the mean phase angle of all the phasors, dashed black line represents the division between phase locked (red), and non-phase-locked ripple phasors. The coupling strength of the ripple phasors recorded from the left amygdala in the NSOZ was comparatively weaker (D2).
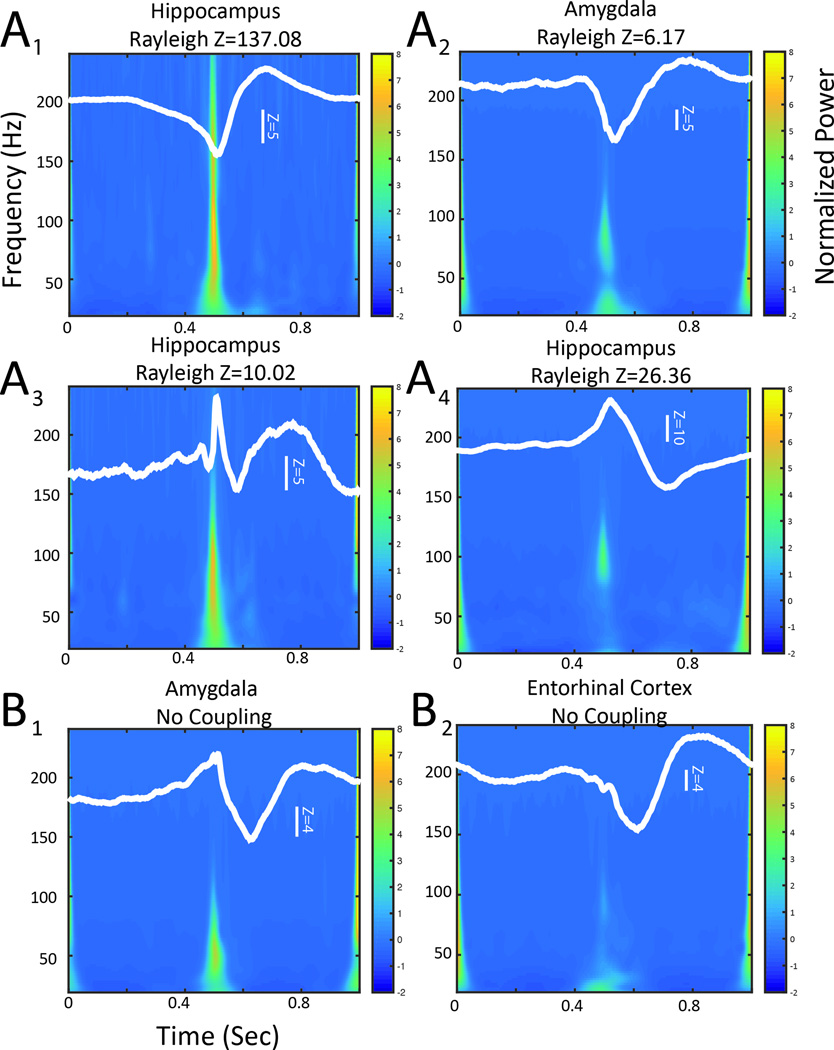
To verify the strength of coupling indicated by the ripple phasor methods, as well as the spectral frequency profile, we calculated the ripple-triggered average to evaluate the modulating signal (i.e. epileptiform spike) and the mean time-spectral frequency representation of the ripples. In the analysis of macroelectrode recordings that exhibited phase-locked ripple phasors, the maximum ripple amplitude occurred near either the peak (Figure 2A1,2) or the trough (Figure 2A3,4) of the epileptiform spike. In 13 out of 16 of the macroelectrodes, gamma amplitude, as well as ripple amplitude was coupled with the epileptiform spike (Figure 2A1–3); however, in the other 3 macroelectrode recordings, only ripple amplitude was coupled to the peak of the epileptiform spike (Figure 2A4). By contrast, in macroelectrode recordings that contained high rates of ripples that were not phase locked with spikes, the corresponding ripple-triggered averaging analysis showed a weak modulating signal (Figure 2B1), and relatively reduced ripple power during the epileptiform spike (Figure 2B2).
Figure 2.
Ripple triggered averaging demonstrates that ripple and gamma amplitude is coupled with the epileptiform spike. The unfiltered iEEG during ripples was temporally aligned to the maximum ripple amplitude, the resulting ripple-triggered averaged signal (white trace) had a peak-to-peak amplitude corresponding to the strength of coupling and a phase at time 0.5 seconds corresponding to the preferred phase of coupling. The superimposed normalized averaged time-frequency representation of the aligned unfiltered ripple events was calculated by wavelet convolution. (A) Ripple triggered averaging results are shown for four electrodes that exhibited phase locked ripple phasors. In example A1 and A2 the preferred phase angle of coupling is near the trough of the epileptiform spike, in examples A3 and A4 the preferred phase angle is near the peak. In A1–A3 epileptiform spike phase is also coupled with gamma amplitude as well as ripple amplitude. B. Ripple triggered averaging results for two electrodes that did not exhibit phase locked ripple phasors. Note the weaker coupling and absence of well-defined ripple power at the preferred phase.
3. Coupling between epileptiform spike phase and ripple amplitude is increased in the seizure onset zone
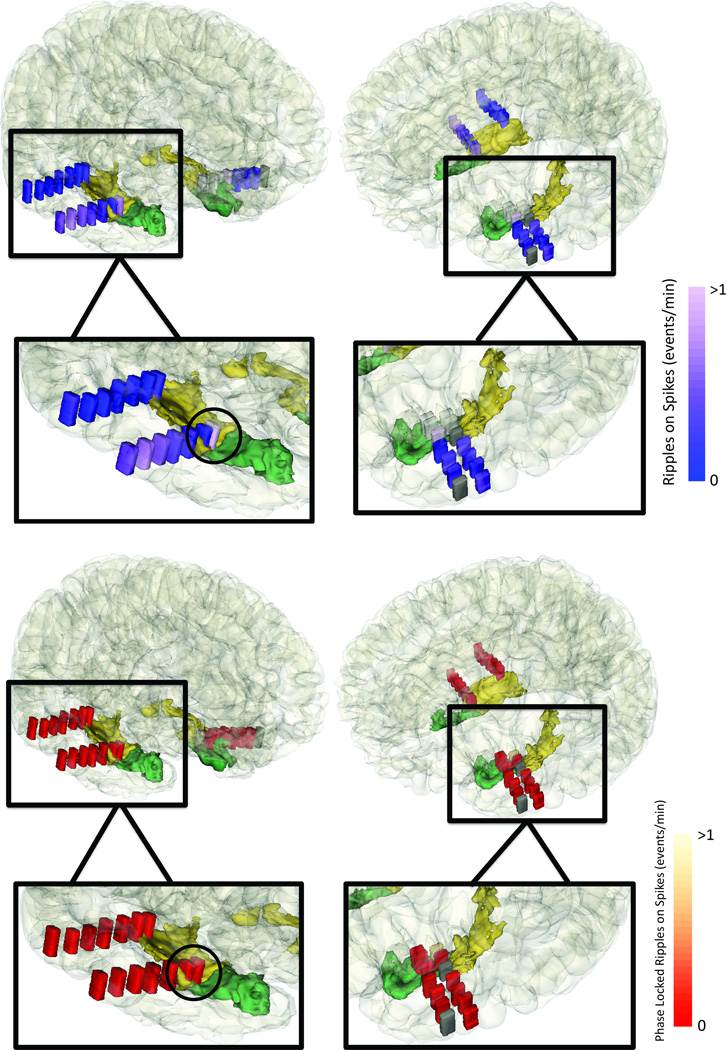
Across the 12 patients, ripple phasors derived from RonS were phase locked in 93 of 147 (63.3%) macroelectrodes in the SOZ, and 137 of 493 (27.8%) of the macroelectrodes in the NSOZ. In certain patients, ripple rates were elevated both in the SOZ and NSOZ, but phase locked ripple rates were elevated exclusively in the SOZ (Figure 3).
Figure 3.
Ripples on spikes that are transformed to phasors can unambiguously lateralize the mesial temporal seizure onset zone. Coregistered CT-MRI of patient #4 with a left mesial temporal seizure onset zone; the rates of ripples on spikes are shown in blue (above), and the rate of the phase locked ripple phasors after transforming the ripples on spikes are shown in red (below). Dark gray macroelectrodes indicate contacts excluded due to poor recording quality.
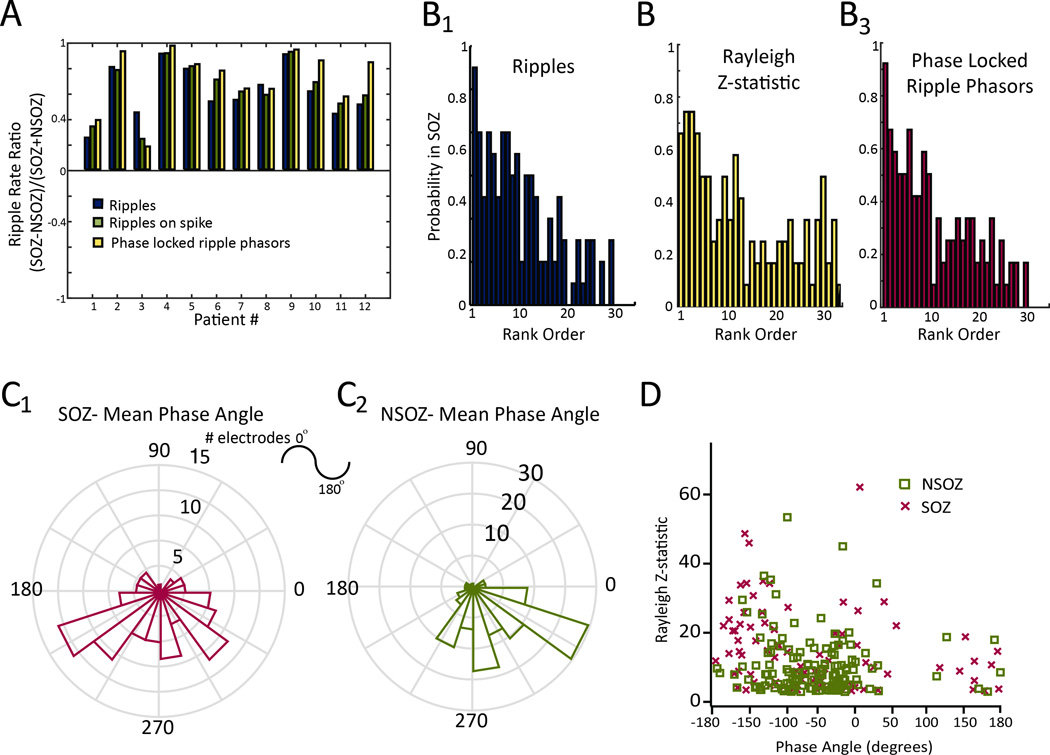
An analysis of ratios of total ripple occurrence in the SOZ to total ripple occurrence in NSOZ showed ratios greater than zero in all patients (Figure 4A). Similarly, ratios derived from the rates of RonS irrespective of coupling were also greater than zero, but in most patients, the highest ratios were those calculated from the phase-locked ripple phasors (Figure 4A, student’s paired t-test, n=12, p<0.05). The rates of total ripples, RonS, and phase-locked ripple phasors were also higher in the SOZ electrodes compared to respective rates in the NSOZ electrodes (Figure S4, student’s paired t-test, n=12, p<0.01). On the basis of visual review of several days of iEEG recording, inter-ictal epileptiform discharges (IEDs) also occurred at a higher rate in the SOZ in the majority of patients (Table 1). However, in patients 6 and 11, IED rates were greater or equal in the mesial temporal lobe electrodes contralateral to the SOZ, respectively; whereas, ripple rates were greatest in the SOZ electrodes.
Figure 4.
Coupling between epileptiform spike phase and ripple amplitude can be used to identify the seizure onset zone. (A) The ripple rate ratio calculated on the basis of mean event rates measured from electrodes in the seizure onset zone (SOZ), and non-seizure onset zone (NSOZ). Both raw ripples, ripples on spikes, and ripples on spikes transformed to phase locked ripple phasors were overly represented in the SOZ in all 12 patients. Across the 12 patients, the SOZ rate ratio for phase locked ripple phasors (yellow) exceeded the ratio for ripples (blue), and ripples on spikes (student’s paired t-test, n=12, p<0.05). (B) Comparison between rank-ordered total rate of ripple occurrence (B1), rank-ordered Rayleigh Z-statistic for the phase-locked ripple phasors (B2), and rank-ordered of rate of phase-locked ripple phasors (B3) and the probability that a given macroelectrode lay within the SOZ. (C) Polar histogram of the mean phase angle of the ripples on spikes transformed to phase-locked ripple phasors measured in the SOZ (red, C1) and the NSOZ (green, C2). D. Scatter plot of the mean phase angle and Rayleigh Z-statistic of these ripple phasors identified in electrodes in the SOZ (red), and in the (NSOZ).
The differences in mean ripple rates in the SOZ and NSOZ does not explicitly assess how the rate at individual electrodes accurately delineates the SOZ. To examine this question in more detail, we correlated the rank-ordered ripple rates with SOZ probability. Analysis showed a higher total ripple rate correlated with increased probability that the electrode was located in the SOZ (R2=0.66, p<0.001, n=30; Figure-5B1). In 11 of the 12 patients, the macroelectrode with the highest ripple rate was observed inside the SOZ (Figure-4B1). Similarly, we evaluated the rank-ordered Rayleigh’s Z-statistic, a measure of phase-locking strength, in relation to the SOZ. We found a larger Z-statistic correlated with increased likelihood the recording sites was in the SOZ (R2=0.44 p<0.001, n=30)(Figure-4B2). Lastly, our analysis showed higher rates of phase-locked ripple phasors, which combine aspects of rate of occurrence and coupling strength, correlated with a greater likelihood the recording sites was in the SOZ (R2=0.67, p<0.001, n=30)(Figure-4B3).
Figure 5.
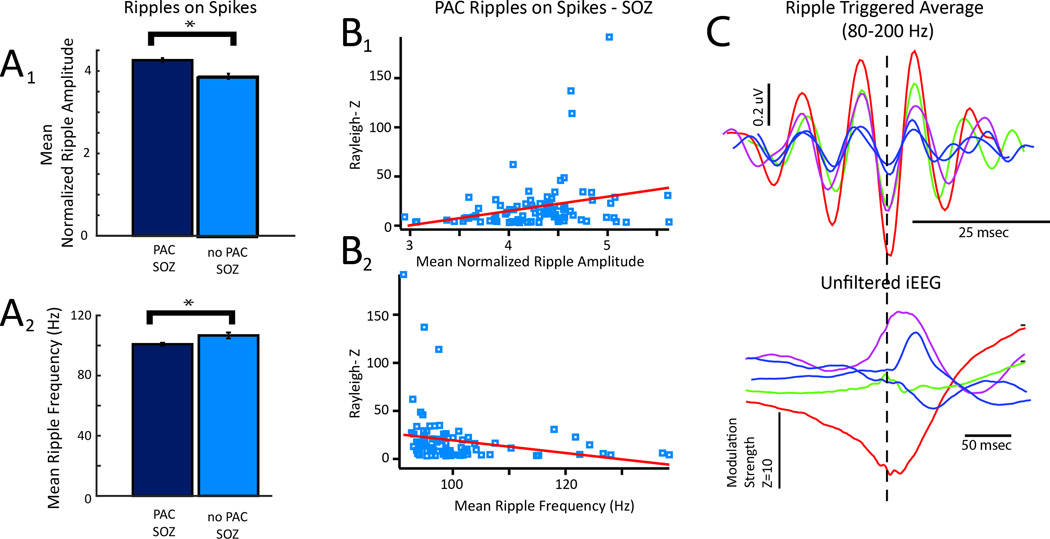
The strength of coupling between epileptiform spike phase and ripple amplitude is positively correlated with mean ripple amplitude and negatively correlated with mean ripple frequency. In recordings from macroelectrodes in the SOZ, the presence of coupling between epileptiform phase and ripple amplitude (PAC) was associated with a greater mean normalized ripple amplitude (A1), and a decreased mean ripple frequency (A2)(student’s unpaired t-test, n=93,54 electrodes, p<0.01, error bars=s.e.m). (B) Among the recordings from macroelectrodes in the SOZ that exhibited PAC the strength of coupling was associated with increased mean normalized ripple amplitude (B1, R2=0.0742, df=92, p<0.01), and decreased mean ripple frequency (B2, R2=0.053, df=92, p<0.05). (C) Illustrative examples of mean ripple triggered averaged waveforms prior to (bottom) and after band-pass filtering (80–200 Hz) top demonstrating relationship between modulation strength and ripple amplitude and frequency.
We also examined if the properties of coupling between the phase of the epileptiform spike and ripple amplitude differed between electrodes located in the SOZ and NSOZ. Polar histograms of the mean phase angle of the phase locked ripple phasors measured across all the macroelectrodes located in the SOZ revealed a preferred phase angle just after the trough of the epileptiform spike and during the upstroke (Figure-4C1); whereas, in the NSOZ, the preferred phase angle approached the peak of epileptiform spike (Figure-4C2). Comparing the Rayleigh Z-statistic with the mean phase angle for each macroelectrode revealed that phase-locking strength was greatest when the mean phase angle was near the peak or the trough of the epileptiform spike (Figure-4D). Across adjacent macroelectrodes positioned in mesial limbic structures, the preferred phase angle of ripple coupling to spikes shifted by over 75° in ~30% of cases. When this phase shift occurred, the ripples recorded by the most-mesial hippocampal and entorhinal cortex contact were coupled with the peak of the epileptiform spike. Phase shifts >150° were observed when comparing the preferred phase angle of coupling measured from contacts in mesial limbic structures with that measured from distal electrodes located in neocortex on the same depth probe in ~66% of cases. No phase shifts were evident for adjacent electrodes positioned in white matter.
3. The strength of coupling between epileptiform spike phase and ripple amplitude influences ripple properties
We asked if the spectral frequency and amplitude of ripples differed between ripples significantly coupled with epileptiform spikes and those that were not coupled. In the SOZ, we found ripples coupled with epileptiform spikes exhibited a greater normalized mean amplitude (4.26 +/− 0.05 vs. 3.87 +/− 0.06, Figure 5A1) and lower mean spectral frequency (100.83 +/− 0.97 vs. 106.71 +/− 1.97 Hz, Figure 5A2), compared to the ripples uncoupled with epileptiform spikes (Student’s unpaired t-test, n=93,54 electrodes, p<0.01). There was no correlation between mean amplitude and spectral frequency (R2=0.0023, df=92, p<0.65). However, increased coupling strength correlated with larger mean normalized ripple amplitude (Figure 5B1, R2=0.0742, df=92, p<0.01), and lower mean ripple spectral frequency (Figure 5B2, R2=0.053, df=92, p<0.05). Finally, ripple-triggered averages showed greater modulation strength of the epileptiform spike correlated with higher ripple amplitude and lower ripple spectral frequency (Figure 5C). In 3 of the 12 patients with both post-resection MRI and outcome data available, we asked if ripples coupled with spikes exhibited different properties in the resected and unresected regions. We found that in patients 2 and 6, with seizure-free outcomes following resection, mean ripple coupling strength was increased, and spectral content was slightly decreased in the resected region, compared to the unresected regions (Figure S5). We did not observe differences in ripple amplitude.
Discussion
The major novel findings from this study show, 1) robust coupling between the phase of epileptiform spikes and the amplitude of ripples, 2) evidence that this form of phase-amplitude coupling is increased in strength in the SOZ, and 3) increased coupling strength correlates with ripples larger in amplitude and of a relatively lower spectral frequency.
Epileptiform spike phase is coupled to ripple amplitude
We used a new ripple phasor method and conventional event-triggered averaging to demonstrate that epileptiform spike phase is coupled to ripple amplitude. Both methods were in general agreement, and found phase amplitude coupling (PAC) in the macroelectrode iEEG recordings.
Coupling between epileptiform spike phase and ripple amplitude is not equivalent to other forms of PAC described in the literature26. For example, in the human neocortex, robust coupling is evident between the phase of theta oscillations and the amplitude of high gamma oscillations. This form of phase amplitude coupling is increased during cognition and decreased in the SOZ27. Theta-high gamma coupling is canonical since both the modulating and the modulated signals are true oscillations. In the case of coupling between epileptiform spike phase and ripple amplitude, the epileptiform spike and ripple are both brief transient events, and furthermore, the spike is not a true oscillation. Coupling due to Gibb’s phenomenon i.e. filter ringing24 is artifactual or non-canonical, since in this case neither the modulated or modulating signals are true oscillations21,22.
The results of the ripple phasor and ripple-triggered averaging methods were also in agreement regarding the preferred phase angle of coupling as near the peak or the trough of the epileptiform spike. This preferred phase angle relates the maximum changes in excitability associated with the inter-ictal discharge to the synchronization of the neuronal assembly generating the pRipple (80–150 Hz). The bimodal distribution of preferred phase angles, near the peak or the trough of the spike, results from the spatial geometry of the dipole relative to the recording electrode. In accord with this concept, we observed shifts of >150° in the preferred phase angle of coupling between mesial contacts and neocortical contacts on the same depth probe. However, we also observed smaller shifts >75° across adjacent electrodes located in mesial temporal structures, suggesting that spike propagation and the cellular and dendritic composition of the tissue adjacent to the macroelectrode contact may also influence the preferred phase angle of coupling.
The ripple phasor method was advantageous in that it was rapid and robust; however, it did not provide information regarding the morphology of the modulating signal or the spectral frequency content of the modulated events. The ripple-triggered averaging method required increased computation, but provided these details.
The ripple-triggered averaging method showed that epileptiform spike phase often modulates ripple, and in some cases gamma, amplitude. The onset of ripple28 and gamma29 oscillations can precede inter-ictal epileptiform discharges, while gamma can occur after inter-ictal discharges as well as fast ripples, i.e. fast ripple tail gamma7. The significance of the gamma oscillations coupled with epileptiform spike phase is unclear, but perhaps may be generated by increased activity among inhibitory interneurons,30 coordinating a large assembly of synchronized principal neurons to generate the pathological ripple (pRipple)16,19. Alternatively, the co-occurrence of ripple and gamma frequencies during inter-ictal discharges may be coincidence, and the mechanisms generating these two frequencies may be independent.
The strength of coupling between epileptiform spike phase and ripple amplitude is increased in the SOZ
We found that the strength of coupling between epileptiform spike phase and ripple amplitude was increased in the SOZ over NSOZ. In addition, compared to ratios derived from total ripples and ripples on spikes (RonS), for most patients larger ratios of phase-locked ripple phasors indicated relatively higher rates of ripples coupled with spikes in the SOZ than rates in NSOZ. Although the differences between ratios were small, phase-locked RonS could improve the localization of SOZ in appropriately powered studies. Since only 2 of the 7 patients who underwent resective epilepsy surgery had a sufficient post-operative observation period, i.e. greater than 12 months, it remains uncertain if ripples, RonS, and coupled RonS rates can also serve as a biomarker of the epileptogenic zone.
Previous studies showed rates of ripples that were associated with epileptiform spikes irrespective of PAC may29,31, or may not be32 superior to rates of ripples in the absence of epileptiform spikes to delineate epileptogenic tissue. Differences between these studies could result from possible contamination of ripples with artifact due to the Gibb’s phenomenon, especially in recordings that contain epileptiform spikes33,34. In the current study, we excluded putative RonS that were most likely produced by filter ringing, and found that in most patients, phase-locked RonS served as a more useful biomarker of the SOZ.
It should also be considered that physiological sharp-wave ripples may have been misclassified as RonS in our study. However, the specificity of the RonS for the SOZ makes this possibility less likely, since sharp wave ripples occur only in the bilateral hippocampi simultaneously19.
While coupling between epileptiform spike phase and ripple amplitude will require additional studies to determine whether it could be used clinically to help localize the SOZ, the direct correlation between the strength of coupling and the probability that a macroelectrode site was in the SOZ has important mechanistic implications, since the coupling may be related to the underlying capacity for synchronization20 among the assembly of neurons generating the pRipple.
Coupling strength influences ripple properties
In recordings from macroelectrodes in the SOZ, greater coupling strength correlated with higher ripple amplitude and lower ripple spectral frequency. It has been demonstrated in studies using computer modeling of normal HFOs35 and optogenetic manipulation of normal tissue16 that the larger the size of the HFO-generating network, the lower the spectral frequency of the HFO. Thus, it is possible in epileptogenic tissue, that if this relationship holds true, when epileptiform spike phase is strongly coupled with ripple amplitude, a large assembly of synchronized neurons could generate a pRipple that contains spectral power between 80 and 150Hz.
The pathologically interconnected neuron (PIN) cluster hypothesis10 predicts that in epileptogenic tissue, very small (1 mm3) clusters of neurons produce synchronized bursts of action potentials that result in fast pRipples (200–600 Hz). It is possible the size of a PIN cluster in epileptogenic sites could expand in the context of changes of excitability. This possibility was clearly established in the context of bathing slices of hippocampus from kainic acid treated rats in the GABAA receptor antagonist bicuculline10. Perhaps the changes in excitability responsible for the epileptiform spike, also cause a massive increase in the size of a synchronized PIN cluster, thereby generating a slower pRipple (80–150 Hz) that occurs at a preferred phase angle. Evidence that ripple frequency oscillations can be pathologic is derived from the findings that they can arise during epileptogenesis in the dentate gyrus that does not usually generate ripple oscillations36, and during ictogenesis, in the hippocampus, entorhinal cortex37, and neocortex38,39.
Clear evidence for synchronized bursting among principal neurons during RonS is lacking. Recordings from single neurons in mesial temporal structures using microelectrodes40–43, or calcium imaging44, have shown that during inter-ictal discharges, just 20–40% of neurons show a change in firing rate40,41,44, that only ~30% of the modulated neurons increase their firing rate or show burst firing40,41, and that across regions of 500µm synchronous firing rarely occurs41. Yet, burst firing in principal neurons is known to be phase-locked to the ripple oscillations42,43, suggesting that synchronized bursting may indeed occur across more restricted spatial regions.
Summary
Results of interictal phase-locked ripples on epileptiform spikes could reflect the fact that epileptogenic regions exhibiting hyperexcitability responsible for epileptiform spikes also synchronize and enlarge clusters of pathologically bursting neurons manifesting as low frequency pRipples. Furthermore, these results are also consistent with the hypothesis that interictal phase-locked ripples on spike are a biomarker of the SOZ.
Supplementary Material
Article Summary.
The phase of epileptiform spikes modulates the amplitude of pathological ripples (pRipples, 80–150 Hz).
The strength of this form of phase amplitude coupling is increased in the seizure onset zone of patients with mesial temporal lobe epilepsy
The strength and rate of coupling improved the precision of the pRipples for identifying the seizure onset zone
The strength of coupling was directly correlated with ripple amplitude and indirectly correlated with ripple spectral frequency.
Changes in excitability cause clusters of pathologically interconnected neurons to expand and synchronize generating pRipples.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
We thank Dr. John Stern, Dr. Dawn Eliashiv, and Dr. Christine Bower-Baca for clinical support, Mr. Kirk Shattuck for technical support, and Ms. Sandra Dewar for administrative support.
Grants: Dr. Weiss is supported by an Epilepsy Foundation Award Research and Training Fellowship for Clinicians, Dr. Orosz by the Otfrid-Foerster grant of the German Epilepsy Society, Miss. Van ‘t Klooster was supported by the Ter Meulen Grant of the Royal Netherlands Academy of Arts and Science (KNAW) and the University Utrecht Short Stay PhD fellowship, Dr. Fried by NINDS grant NS033221, Dr. Engel by NS033310, Dr. Staba by NS071048, and Dr. Bragin by NS065877.
Biography

Dr. Shennan Aibel Weiss is an Assistant Professor in the Department of Neurology at Thomas Jefferson University, he recently completed his epilepsy fellowship at the University of California Los Angeles.
Footnotes
Conflict of Interest: None related to this article
References
- 1.Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44:741–751. doi: 10.1046/j.1528-1157.2003.48202.x. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe S, Blume WT, Girvin JP, et al. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 3.Engel J, Jr, McDermott MP, Wiebe S, et al. Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy: A Randomized Trial. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel J, Jr, Bragin A, Staba R, et al. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 5.Gotman J. High frequency oscillations: The new EEG frontier? Epilepsia. 2010;51:63–65. doi: 10.1111/j.1528-1167.2009.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 7.Bragin A, Engel J, Jr, Wilson C, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 8.Staba RJ, Wilson CL, Bragin A, et al. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 9.Bragin A, Wilson CL, Staba RJ, et al. Interictal high-frequency oscillations (80–500Hz) in the human epileptic brain: Entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 10.Bragin A, Mody I, Wilson CL, et al. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 12.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 14.Lachaux J-P, Axmacher N, Mormann F, et al. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ylinen A, Bragin A, Nadasdy Z, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark E, Roux L, Eichler R, et al. Pyramidal Cell-Interneuron Interactions Underlie Hippocampal Ripple Oscillations. Neuron. 2014;83:467–480. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs J, Staba R, Asano E, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98:302–315. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haegelen C, Perucca P, Chatillon CÉ, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54:848–857. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive marker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canolty RT, Ganguly K, Kennerley SW, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci U S A. 2010;107:17356–17361. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss SA, Banks GP, McKhann GM, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. 2013;136:3796–3808. doi: 10.1093/brain/awt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss SA, Lemesiou A, Connors R, et al. Seizure localization using ictal phase-locked high gamma: A retrospective surgical outcome study. Neurology. 2015;84:2320–2328. doi: 10.1212/WNL.0000000000001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain. 2014;137:183–196. doi: 10.1093/brain/awt299. [DOI] [PubMed] [Google Scholar]
- 24.Bénar CG, Chauvière L, Bartolomei F, et al. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on"false” ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak D, Fenton AA. Toward a proper estimation of phase–amplitude coupling in neural oscillations. J Neurosci Methods. 2014;225:42–56. doi: 10.1016/j.jneumeth.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canolty RT, Edwards E, Dalal SS, et al. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Klink N, Frauscher B, Zijlmans M, et al. Relationships between interictal epileptic spikes and ripples in surface EEG. Clin Neurophysiol. 2016;127:143–149. doi: 10.1016/j.clinph.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 29.Ren L, Kucewicz MT, Cimbalnik J, et al. Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology. 2015;84:602–608. doi: 10.1212/WNL.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muldoon SF, Villette V, Tressard T, et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain. 2015;138:2875–2890. doi: 10.1093/brain/awv227. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Wang IZ, Bulacio JC, et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54:370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs J, LeVan P, Chander R, et al. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amiri M, Lina J-M, Pizzo F. High Frequency Oscillations and spikes: Separating real HFOs from false oscillations. Clin Neurophysiol. 2016;127:187–196. doi: 10.1016/j.clinph.2015.04.290. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs J, Vogt C, LeVan P, et al. The identification of distinct high-frequency oscillations during spikes delineates the seizure onset zone better than high-frequency spectral power changes. Clin Neurophysiol. 2016;127:129–142. doi: 10.1016/j.clinph.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 35.Brunel N, Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- 36.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2003;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 37.Weiss SA, Alvarado-Rojas C, Bragin A, et al. Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia. 2016;57:111–121. doi: 10.1111/epi.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnatkovsky V, de Curtis M, Pastori C, Cardinale F, Lo Russo G, Mai R, Nobili L, Sartori I, Tassi L, Francione S. Biomarkers of epileptogenic zone defined by quantified stereo-EEG analysis. Epilepsia. 2014;55:296–305. doi: 10.1111/epi.12507. [DOI] [PubMed] [Google Scholar]
- 39.Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 40.Keller CJ, Truccolo W, Gale JT, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133:1668–1681. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarado-Rojas C, Lehongre K, Bagdasaryan J, et al. Single-unit activities during epileptic discharges in the human hippocampal formation. Front Comput Neurosci. 2013;7:140. doi: 10.3389/fncom.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Van Quyen M, Bragin A, Staba R, et al. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarado-Rojas C, Huberfeld G, Baulac M, et al. Different mechanisms of ripple-like oscillations in the human epileptic subiculum. Ann Neurol. 2015;77:281–290. doi: 10.1002/ana.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muldoon SF, Villette V, Tressard T, et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain. 2015;138:2875–2890. doi: 10.1093/brain/awv227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.