Abstract
In approximately 30% of patients with epilepsy, seizures are refractory to medical therapy, leading to significant morbidity and increased mortality. Substantial evidence has demonstrated the benefit of surgical resection in patients with drug-resistant focal epilepsy, and in the present journal, we recently reviewed seizure outcomes in resective epilepsy surgery. However, not all patients are candidates for or amenable to open surgical resection for epilepsy. Fortunately, several non-resective surgical options are now available at various epilepsy centers, including novel therapies which have been pioneered in recent years. Ablative procedures such as stereotactic laser ablation and stereotactic radiosurgery offer minimally invasive alternatives to open surgery with relatively favorable seizure outcomes, particularly in patients with mesial temporal lobe epilepsy. For certain individuals who are not candidates for ablation or resection, palliative neuromodulation procedures such as vagus nerve stimulation, deep brain stimulation, or responsive neurostimulation may result in a significant decrease in seizure frequency and improved quality of life. Finally, disconnection procedures such as multiple subpial transections and corpus callosotomy continue to play a role in select patients with an eloquent epileptogenic zone or intractable atonic seizures, respectively. Overall, open surgical resection remains the gold standard treatment for drug-resistant epilepsy, although it is significantly under-utilized. While non-resective epilepsy procedures have not replaced the need for resection, there is hope that these additional surgical options will increase the number of patients who receive treatment for this devastating disorder - particularly individuals who are not candidates for or who have failed resection.
Keywords: brain stimulation, disconnection, epilepsy surgery, review, seizure outcome
Introduction
Uncontrolled seizures lead to significant morbidity and increased mortality, but patients with drug-resistant epilepsy may be candidates for epilepsy surgery [22,35]. Established guidelines recommend that patients with epilepsy who have failed two or more anti-epileptic drug trials are unlikely to achieve seizure-freedom with further medication changes alone, and should thus be referred to a comprehensive epilepsy center for surgical evaluation [82,24,35]. In mesial temporal lobe epilepsy (MTLE) and focal neocortical epilepsy (FNE), localization and resection of the epileptogenic zone (EZ) is the gold standard surgical treatment in both children and adults [44,49,116,51,40], and the use of anterior temporal lobectomy for drug-resistant MTLE is supported by two randomized-controlled trials [34,130]. In the present journal, we recently provided an update of rates and predictors of seizure freedom with resective epilepsy surgery across recent literature [41]. However, not every patient with drug-resistant epilepsy is a candidate for or amenable to surgical resection, so a thorough understanding of non-resective surgical alternatives is imperative in the treatment of this disorder. Thus, the goal of the present review is a concise yet comprehensive summary of seizure outcomes after non-resective surgical procedures for epilepsy, including a critical overview of recent important literature.
While most studies investigate patients who have not received prior open surgical resection for epilepsy, we also reference manuscripts examining outcomes of non-resective surgery after failed resection, given that re-operation represents another aspect of surgical care [37]. While the goal of epilepsy resection is to excise abnormal region(s) of the brain harboring an EZ in hopes of achieving seizure freedom, patients with generalized epilepsy syndromes or seizure foci that are poorly localized, multifocal, or positioned in eloquent brain regions are often not candidates for resection [41,16]. For these individuals, palliative surgical options may be considered to reduce the frequency and severity of seizures, and these include stimulation-based therapy using vagus nerve stimulation (VNS), deep brain stimulation (DBS), and responsive neurostimulation (RNS), as well as disconnection procedures such as multiple subpial transections (MST) and corpus callosotomy (CC). Unlike disconnective surgeries, each of the three stimulation treatment strategies has been evaluated in randomized, controlled trials. Furthermore, novel techniques for EZ ablation, such as stereotactic laser ablation (SLA) and stereotactic radiosurgery (SRS), now allow minimally invasive surgical options for select individuals who are poor candidates for open surgery, or are simply averse to it. Importantly, reporting of outcome measures in the literature often differs somewhat between interventions. As with resection, the ultimate goal of ablation is typically complete seizure freedom, and thus seizure freedom rates are the primary outcome measure reported for ablative interventions. However, complete seizure freedom is rare after palliative neurostimulation, and therefore percent decrease in seizure frequency and rate of response to therapy (defined as ≥50% decrease in seizures) are typically reported as primary outcome measures for these procedures. Finally, callosotomy studies usually report reduced frequency of drop attacks.
Ablative Procedures
The primary objective of ablative procedures for intractable focal epilepsy resembles the goal of resective strategies: destruction of epileptogenic tissue to prevent further seizures. Two ablative therapies for epilepsy pioneered in recent years, SLA and SRS, lead to tissue necrosis using thermal energy or radiation, respectively. In general, these procedures offer a minimally invasive alternative to craniotomy for patients with significant risk factors for open surgery.
Stereotactic laser ablation (SLA)
Radiofrequency thermoablation has been used for focal stereotactic brain lesioning in the treatment of intractable epilepsy. In one series of 22 patients with MTLE, stereotactic radiofrequency amygdalohippocampectomy resulted in complete seizure freedom in two (9%) patients, and worthwhile improvement was observed in an additional 8 (25%) individuals [97]. Case reports examining the use of radiofrequency ablation for hypothalamic hamartomas have revealed favorable seizure outcomes in some patients, and 3 of 5 (60%) individuals treated achieved seizure freedom in one small case series [75]. Radiofrequency ablation has also been reported in patients implanted with diagnostic stereotactic depth electrodes, utilizing recording electrodes to lesion a confirmed epileptogenic region [63,62]. Finally, radiofrequency ablation for periventricular heterotopia has also been described with some success [112]. However, given improvements in MRI thermometry, and challenges related to monitoring and controlling radiofrequency lesions, interest in real-time stereotactic ablation using laser thermal energy has recently increased[118,61].
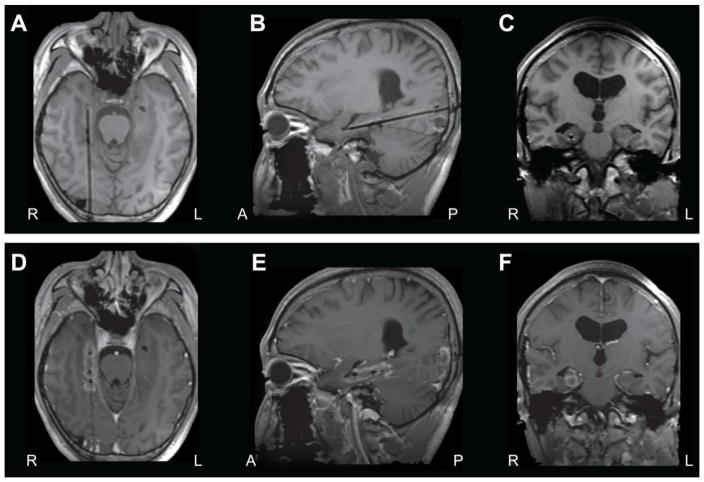
The SLA procedure can be performed with a small scalp incision and miniature burr hole, with a laser probe placed using a stereotactic frame or interventional MRI techniques. The most interest in SLA for epilepsy has been focused on selective lesioning of the amygdalo-hippocampal complex in MTLE [70,133,127]. Approaching the hippocampus from an occipital trajectory along its longitudinal axis allows access to a large portion of the structure, while the inferior horn of the lateral ventricle (laterally) and the basal cisterns (medially) create a “heat sink” that helps prevent thermal injury to nearby structures and vasculature [60,134]. MRI images from an example case of SLA for MTLE are shown in Figure 1.
Figure 1. Stereotactic laser ablation (SLA) for mesial temporal lobe epilepsy.
A–C) Shown are T1-weighted MRI axial (A), sagittal (B), and coronal (C) images showing during laser probe placement along the axis of the left hippocampus, prior to SLA in a patient with mesial temporal lobe epilepsy. D–F) Contrast-enhanced T1-weighted MRI axial (D), sagittal (E), and coronal (F) images obtained approximately 5–10 minutes after thermal ablation of mesial temporal lobe structures, with contrast enhancement seen in the region of ablation. Lesioning is performed with real-time MRI thermal measurements. A: anterior; L: left; P: posterior; R: right.
Willie and colleagues reported a series of 13 adult patients who received SLA for MTLE with or without mesial temporal sclerosis [133]. The authors reported a mean volume of 60% ablation of the amygdalohippocampal complex, and a median hospitalization of only one day. After 5 to 26 months of follow-up, with a median of 14 months, 54% of patients were free of disabling seizures, and 67% (6 of 9) of individuals with mesial temporal sclerosis were seizure free. Seizure recurrences were all observed within the first 6 months, and differences in ablation volume or length did not account for clinical outcomes. There was one significant adverse event involving a visual field defect caused by deviated insertion of a stereotactic aligning rod, although the alignment was corrected prior to ablation [133]. A follow-up study by the same group examined certain neuropsychological outcomes in 19 patients undergoing SLA for MTLE, compared to 39 individuals undergoing standard resection, using a prospective, nonrandomized, parallel-group design [31]. Compared to open surgical resection, it was found that SLA was associated with significantly improved naming in patients with dominant hemisphere MTLE, and better object recognition in individuals with MTLE of the nondominant hemisphere. Overall, no patients showed decline in performance in naming and object recognition tasks after SLA, suggesting that the hippocampus does not play an essential role in these neural networks [31]. Another group examined SLA outcomes in 20 patients with MTLE and measured ablation volumes [77]. Seizure freedom was reported in 53% of 15 patients at 6 months, 36% of 11 patients at one year, and 60% of 5 individuals at two years (median follow-up, 13 months). No differences were noted in total ablated volumes of hippocampus, amygdala, parahippocampal gyrus, entorhinal cortex, and fusiform gyrus in patients who did or did not achieve seizure freedom.
SLA outcomes have also been examined in pediatric patients with various epilepsy etiologies [15]. In a recent series of 19 pediatric patients receiving SLA for intractable epilepsy, Lewis et al. reported seizure freedom in 41% of individuals after a mean follow-up of 16 months (range, 4 to 36) [84]. Nearly all of these patients suffered from FNE, with epilepsy etiology in 11 individuals consistent with focal cortical dysplasia, and 10 patients had received prior resection. Thus, as with resective surgery, seizure outcomes with SLA for FNE appear inferior to outcomes in the treatment of MTLE.
Another study reported the use of SLA to treat cavernous malformations associated with drug-resistant epilepsy in five patients, observing seizure freedom in 4 (80%) individuals 12 to 28 months after treatment [89]. Furthermore, some groups have successfully used SLA to treatment hypothalamic harmartomas associated with epilepsy – a lesion that is particularly challenging to access safely with open surgery [131]. These reports suggest that SLA may be a feasible treatment alternative to resection with other epileptogenic lesions beyond mesial temporal lobe structures.
Overall, early patient series suggest relatively favorable outcomes with SLA for patients with focal epilepsy – particularly those with MTLE – although seizure freedom rates are lower than those with traditional resection. Nonetheless, the minimally invasive nature of this procedure may make it a desirable treatment option for patients who are averse to resection, or those with significant surgical risk factors. Further studies are needed to define long-term seizure and neuropsychological outcomes after SLA, and to better elucidate the role and efficacy of surgical resection in patients who have failed ablation as an initial operative therapy. Furthermore, no previously published SLA studies have specifically examined patients who failed prior epilepsy surgery, but such investigations will be important going forward.
Stereotactic radiosurgery (SRS)
SRS for MTLE is the only non-resective procedure for epilepsy which completely avoids invasive surgery. SRS is performed with gamma knife targeted radiation, which uses radioactive cobalt to deliver 192 beams of radiation to a targeted area of the brain in a single fraction while preserving surrounding parenchyma [103,102,59]. Similar to SLA, SRS may be a favorable option for patients with MTLE who refuse resection or have medical comorbidities that increase peri-operative risk. Early studies have examined the safety and efficacy of SRS for MTLE patients with mesial temporal sclerosis [103,104,7]. In general, seizure outcomes in these reports have been relatively comparable to open temporal lobectomy, particularly in patients receiving high dose therapy (24 Gy) to the amygdala, hippocampal head, and parahippocampal gyrus.
Barbaro et al reported a pilot multi-centered prospective trial of SRS for MTLE, and observed seizure freedom in 77% of 13 patients who received high dose (24 Gy) treatment and in 59% of 17 individuals who received low dose (20 Gy) therapy one year after the procedure [7]. This data suggests statistically improved efficacy of high dose compared to low dose SRS for MTLE. Among these patients, verbal memory impairment was noted in 15% of patients, although none declined on more than one measure, while verbal memory improvement was seen 12% of individuals [7,101]. Side effects were minimal, and included transient steroid requirements, headache, and visual field deficits. One individual suffered from malignant edema after treatment, including severe headaches, visual field deficit, and papilledema not responsive to steroids, and this patient eventually required temporal lobectomy [7]. The final results are awaited from a prospective randomized trial of SRS versus open temporal lobectomy (Radiosurgery or Open Surgery for Epilepsy [ROSE] trial) [107]. Of note, while most SRS epilepsy studies examine patients who have not undergone prior intervention, one small series reported SRS outcomes in four patients who failed open temporal lobectomy[137]. With a follow-up of 19–24 months, these patients had a 42% mean decrease in seizure frequency (range 28–67%) after SRS, but no patients were completely free of seizures.
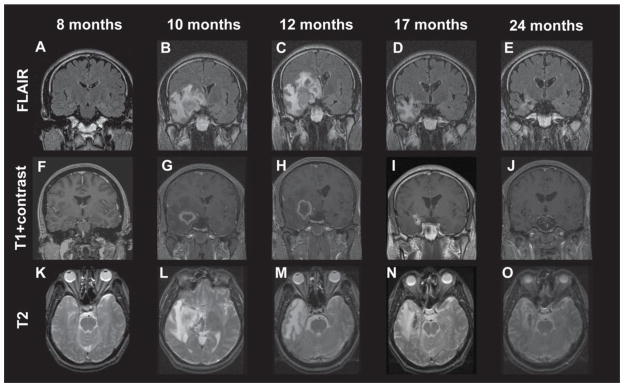
Importantly, unlike resection, the beneficial effects of SRS on seizures in MTLE are typically delayed up to 12 months or more after treatment. Chang and colleagues found that MRI characteristics during the first year following SRS may serve as a predictor of seizure outcome at three years after therapy [18]. Specifically, T2 hyperintensity volumes nine months after the procedure were found to be highly related to seizure remission, and were more pronounced in patients who received 24 Gy SRS compared to lower dose 20 Gy treatment [7,17]. Spectroscopy suggested a mechanism of action consistent with radiation necrosis, revealing that the treatment indeed results in tissue destruction. The development of these radiographic changes over time after SRS are summarized in Figure 2. Thus, compared to SLA, which results in immediate tissue necrosis from thermal damage (eg., Fig. 1), SRS results in delayed radiation necrosis. Similarly, the clinical benefits of SRS on seizure status are also delayed, compared to the immediate clinical effects observed after SLA. Future investigations will need to evaluate long-term seizure and cognitive outcomes in SRS compared to resection and SLA.
Figure 2. Development of radiologic changes in a patient with mesial temporal lobe epilepsy treated with a 24-Gy dose Gamma Knife stereotactic radiosurgery (SRS).
FLAIR (A–E) and T2 (K–O) hyperintensity appeared within the medial temporal lobe beginning by the 10th postoperative month and peaked in intensity at 12 months, corresponding to a decline in the proportion of patients experiencing complex partial seizures. Contrast enhancement (F–J) followed a similar time course, except that it preceded T2 changes and diminished quickly after months 10–12. Enhancement was typically ring-enhancing and centered over the target region. Figure and legend reproduced with license and permission from Chang et al. [17].
Neurostimulation Procedures
While seizure freedom is usually the goal of resective and ablative procedures for epilepsy, device implantation for neurostimulation in epilepsy typically represents a palliative treatment approach. Complete seizure freedom is uncommon with current neuromodulation technologies, and thus reduction of seizure frequency and morbidity, as well as improvement in quality of life, are the primary treatment goals. The three neurostimulation options for epilepsy which have been well-studied include VNS, DBS, and RNS.
Vagus nerve stimulation (VNS)
Approved by the United States Food and Drug Administration (US FDA) in 1997 and adopted in more than 70 countries, VNS is utilized for localization-related epilepsy with multiple or non-resectable foci, after unsuccessful intracranial epilepsy operations, and in generalized epilepsy syndromes [27,53]. The primary delivery mechanism of VNS is a neuro-cybernetic prosthesis, and animal and human studies suggest that vagal stimulation may lead to desynchronization and decreased abnormal spike activity on EEG by enabling nonselective and bidirectional activation of nerve fibers [74,79,19]. Currently, over 100,000 VNS devices have been implanted worldwide [27].
Three blinded, randomized controlled trials have shed light on VNS efficacy for medically refractory epilepsy. First, Ben-Menachem and colleagues performed a randomized study with 114 focal epilepsy patients who received either therapeutic or sham stimulation after VNS implantation. They reported a significantly greater reduction in seizure frequency with therapeutic stimulation after three months of treatment (25% versus 6%) [10]. Handforth and colleagues also led a multi-centered trial of 196 patients with partial-epilepsy, and observed a 28% reduction in seizure frequency with high stimulation versus 15% with sham stimulation [67]. Overall, 23% of individuals in the therapeutic group reaching ≥50% seizure reduction at three months. Next, Amar et al. observed more dramatic results in a small VNS trial including 17 patients, within which 57% of individuals receiving therapeutic stimulation achieved ≥50% decrease in seizure frequency [3]. Two non-blinded, randomized trials have also reported significant decreases in seizure frequency with various VNS stimulation paradigms [28,110].
Although these randomized-controlled trials of VNS included only a short follow-up period, observational studies have demonstrated improved efficacy over time with treatment. One large meta-analysis of patients treated with VNS included 3,321 patients from 77 reports. Reduction in seizure frequency as compared to the baseline indicated that 51% of patients treated with VNS obtained ≥50% seizure frequency [43]. Seizure control rates rose as therapy duration increased, although few patients achieved complete seizure freedom. Similar outcomes were observed through unblinded analysis of the device manufacturer’s patient database [42]. Favorable response to therapy, with ≥50% reduction in seizure frequency, was reached by 1972 of 4483 (44%) patients after three months of therapy, and by 618 of 1104 (56%) patients after 24 months. Patients with a history of Lennox-Gastaut syndrome or epilepsy stemming from a traumatic source may benefit from an improved treatment response [50]. Adverse events associated with VNS include hoarseness (37–62%), cough (7–21%), pain (6–17%), and infection (4–6%) [67,29,10,42]. A Cochrane systematic review of VNS efficacy and tolerability for partial seizures among 439 patients was also recently conducted. The four trials analyzing high-level stimulation compared to low-level stimulation were summarized, and together revealed an overall risk ratio of 1.73 for 50% or greater seizure reduction [96].
One study of the VNS Therapy Patient Outcome Registry compared seizure outcomes after VNS in 921 patients who failed prior resection, compared to 3,822 individuals who did not have previous surgery [2]. The median reduction in seizure frequency at 24 months was lower in patients who had failed previous surgery (51%) compared to those who had not (67%) undergone prior resection. A responder rate of 55% vs. 62% was seen in these two groups, respectively, at last follow-up. These data suggest that individuals who have failed epilepsy surgery may have less favorable VNS outcomes than patients without a history of resection, but may nonetheless experience worthwhile improvement in seizure frequency.
Finally, while complete seizure freedom is relatively rare with VNS, seizure freedom rates and predictors were recently examined across 5,554 patients in the VNS Therapy Patient Outcome Registry, and in a systematic literature review of 78 studies including 2,869 patients [52]. Overall, seizure-freedom rates ranged from 3% after zero to four months of treatment to 8% after more than two years of therapy. Predictors of seizure freedom included age of epilepsy onset of greater than 12 years and a predominantly generalized seizure type [52]. Overall, when resective epilepsy surgery is not a viable treatment option, or in individuals who have failed open surgery, VNS may represent worthwhile palliative therapy for pharmacoresistant epilepsy. However, patients and providers must recognize that substantial clinical benefit (> 50% decrease in seizures) is only experienced in 50–60% of patients who receive VNS, and maximum seizure reduction may require 1–2 years of stimulation.
Deep brain stimulation (DBS)
In the mid-20th century, the cerebellum was identified as a potential stimulation target for intractable epilepsy, thought to cause thalamic inhibition by increasing Purkinje cell output, but the degree of benefit was unclear due to nonblinded studies with unquantified outcomes [93,23]. Hippocampal DBS has also been proposed in the treatment of epilepsy, in part given the effects of stimulation on desynchronization of mesial temporal networks [122]. Animal models of hippocampal DBS for epilepsy have demonstrated moderate efficacy [66]. In a kainic acid macaque primate epilepsy model, hippocampal stimulation has been shown to be protective again neuronal apoptosis [20], and high frequency stimulation of the hippocampus in kindled rats has been associated with higher threshold and longer latency of after-discharges [135]. Nevertheless, randomized-controlled trial of hippocampal DBS in humans has not yet been reported [66,120].
The subthalamic nucleus is a common DBS target for Parkinson’s disease, and some investigators have examined subthalamic stimulation for epilepsy, hypothesizing that basal ganglia modulation may impact epileptogenicity [83]. Specifically, a few reports have found decreased seizure frequency in a small number patients with myoclonic epilepsy, but a large-scale investigation has never been pursued with this target [126,132]. Given its role in cortical activation, DBS of the centromedian nucleus of the thalamus has also been explored in refractory epilepsy. Velasco and colleagues reported reduced rates of generalized seizures and improved quality of life in 13 patients with Lennox-Gastaut syndrome [125]. Another group evaluated centromedian nucleus stimulation versus sham treatment in six patients with intractable seizures, and observed a 30% decrease in seizures with stimulation, although this difference was not significant [56]. These and preliminary results from other groups [4,123] suggest that the centromedian nucleus may be a target worthy of further study. Finally, beyond decreasing seizure frequency, rodent studies have suggested that thalamic stimulation may prevent aberrant network effects of seizures that lead to ictal impairment of consciousness [65], perhaps by effecting networks involved in normal cortical activation [46,45]. However, human studies examining ictal effects of thalamic stimulation have not yet been performed.
In DBS for epilepsy, recent focus has turned to the anterior nucleus of the thalamus, a structure in the classic circuit of Papez intimately connected to limbic structures, and with widespread neocortical projections [106]. Thalamic DBS has been approved as adjunctive treatment for medication-resistant epilepsy as well as secondary generalized seizures in Canada, the European Union, Australia, Taiwan, New Zealand, and Australia, but it is not yet approved by the US FDA [8,53]. While the mechanisms underlying DBS in general remain unknown, many have suggested that high-frequency stimulation (>50 Hz) produces a reversible lesion that mimics ablation [83].
Thalamic DBS was studied in 110 adults with medication-resistant partial epilepsy with or without secondary generalization in the randomized Stimulation of the Anterior Nucleus of Thalamus for Epilepsy (SANTE) trial [54]. During the three-month blinded period of the SANTE trial, individuals receiving stimulation showed a significantly greater decrease in seizure frequency (40%) compared to those in the control arm of the study (15%). During the unblinded phase of the study, within which all patients were treated with stimulation for two years, seizure frequency was reduced by 56% (median), and 54% of patients experienced a reduction in seizure frequency of ≥50%. A trend was observed towards improved seizure control with longer stimulation periods, as has been seen with other neurostimulation treatments such as VNS [42]. Recently, long-term outcomes have been reported by the SANTE investigators, revealing a median percent seizure reduction of 69% and responder rate of 68% five years after surgery [109]. This suggests that a beneficial neuromodulatory effect of DBS may extend beyond the first few years of therapy.
During the first year of thalamic DBS, adverse events include paresthesias in 18% of individuals, surgical site pain in 11%, local infections in 9%, and need for lead replacement in 8%, although these complication rates decreased in the second treatment year [54]. The overall rate of serious side effects secondary to the treatment was l-2%. Neuropsychological testing revealed no differences in mood or cognitive between treated and untreated patients, but individuals receiving stimulation were more likely to report symptoms of depression. Given that rates of seizure control after thalamic DBS years versus VNS are relatively similar one to two years after implantation, a detailed comparative investigation of palliative neurostimulation procedures among patient subpopulations will likely be informative. Furthermore, DBS outcomes in epilepsy patients who have failed epilepsy resection will be an interesting topic for future exploration, given the absence of literature on this topic.
Responsive neurostimulation (RNS)
While VNS and DBS use open-loop stimulation paradigms with uninterrupted electrical pulses, RNS utilizes a closed-loop stimulation system [106]. Implanted subdural and depth electrodes continuously record neurophysiological signals during ictal events, and these data can be analyzed offline for programming of the device. Then, stimulation is triggered by electrographic activity concerning for seizure initiation, designed to terminate the epileptic discharge before it becomes symptomatic [92]. The RNS device, which is depicted in Figure 2, received approval by US FDA in 2013 for the treatment of adults with intractable partial epilepsy [53]. While VNS and DBS do not require a hypothesis regarding EZ localization, and indeed may be used in generalized epilepsy syndromes, RNS does require knowledge regarding seizure localization in order to effectively deliver targeted therapy. With current technology, up to two regions can be simultaneously targeted for active therapy with RNS. Thus, RNS is typically pursued in patients with multiple EZs – such as bilateral mesial temporal seizure onset – or an EZ located in eloquent cortex not amenable to resection [78,16].
The efficacy of RNS was first examined in a multicentered, randomized, double-blinded, controlled trial termed the RNS System Pivotal Trial [92]. In this trial, 191 adult patients with drug-resistant partial epilepsy received implantation of the RNS system and were randomized to receive responsive stimulation versus seizure detection without stimulation during a 12-week blinded period. Individuals receiving stimulation experienced a reduction in seizure frequency of 38%, versus 17% in the sham-treatment group. Also, 29% patients receiving stimulation reported ≥50% reduction in seizures, while this outcome was reported in 27% of control subjects. After three months of randomization, individuals in both groups then received therapeutic stimulation. In this open study phase, 44% or 55% of individuals experienced ≥50% reduction in seizure frequency at one or two years, respectively. Furthermore, the median percent seizure reduction was 44% at one year and 53% at two years in these patients[71]. While these findings suggest improved seizure control over time with RNS, it is important to recognize that RNS outcomes beyond 3 months do not reflect randomized controlled data. Adverse events in the trial included hardware site infection (5.2%), headache (10.5%), dysesthesia (6.3%), increase in generalized (4.7%) or complex-partial (5.8%) seizures, and other complications were rare [92,71]. Serious adverse event rates did not differ between patients receiving therapeutic or sham stimulation.
Recently, long-term outcomes with RNS have also been reported [12]. During post-implant years 3 to 6, median percent seizure reduction ranged from 48 to 66%, although the responder rate remained relatively unchanged at 59–61%. Overall, data do suggest a trend toward improved seizure outcome over time with RNS [12,71]. After 5.4 years mean follow-up, the most common treatment-related complications included device site infection involving soft tissue (9%) and explantation of the neurostimulator (5%) [12]. Neuropsychological outcomes have also been examined in these RNS trial patients. No significant neurocognitive declines have been reported during the first two years of treatment, and improved neuropsychological parameters were observed in some instances [85]. Specifically, small improvements in naming abilities were reported in patients with FNE, while small improvements in verbal learning were observed in individuals with MTLE. No new adverse changes in mood have been reported in this RNS patient population, and 44% of individuals reported meaningful improvement in quality of life after two years of treatment, compared to 16% of patients who reported a decline [90].
Overall, RNS appears safe, and many patients do experience clinical improvement after one to two years of therapy, although benefits may be marginal in the first few months of treatment. Tailored responsive stimulation therapy for epilepsy is an important area of research, given potential benefits over open-loop stimulation treatments, such as fewer side effects and improved device battery life [92,55]. Furthermore, RNS outcomes in patients who have already failed resective surgery will require further study. Broader clinical use of RNS will be dependent on further study and continued improvements in this technology.
Disconnection Procedures
Even prior to laser or radiosurgical ablation techniques, or the development of neurostimulation devices for neuromodulation, disconnection procedures for epilepsy have been explored, with the goal of limiting seizure spread and reducing morbidity. Two relatively common disconnection procedures that continue to be utilized in many epilepsy surgery practices include MST and CC. Large-scale disconnection procedures for catastrophic hemispheric epilepsy syndromes, such as functional hemispherectomy or hemispherotomy, are not included here, but have been recently discussed [41]. Complete seizure freedom is rare with disconnection procedures alone.
Multiple subpial transections (MST)
MST was introduced by Morrell in 1989 as a targeted disconnective procedure for patients with an EZ localized to eloquent neocortex, such as that subserving speech, vision, and primary motor and sensory function [91]. The procedure involves numerous parallel subpial incisions applied to involved cortex to severe tangential intracortical fibers, and is based on evidence suggesting that epileptic spread requires horizontal cortico-cortico connections, while most functional neuronal signals travel vertically in cortical columns [91,6]. Modification of the original technique, utilizing radiating MST with a single cortical incision, guided by electrocorticography and neuronavigation, has also recently been described [94]. While eloquent cortex is typically targeted with MST, multiple hippocampal transection has also been described as a potentially verbal memory-preserving surgical approach for the treatment of unilateral MTLE [99].
MST has been associated with low risk of neurological compromise [117,136], but its adoption as a tool for seizure control has been inconsistent. In 2002, Spencer and colleagues performed an analysis of 211 patients undergoing MST at six centers, and found a 62–71% obtained an “excellent” seizure outcome (> 95% reduction in seizure frequency) with the use of MST alone, and 87% of patients achieved this outcome when MST was combined with resection [117]. No significant response predictors were identified in this study. Of note, other groups have described more modest results, particularly in children, with 33–46% of patients reaching Engel class 1 or II outcome after MST without associated resection [100,11,13]. Recently, Downes and colleagues observed no difference in seizure status among epilepsy patients with Landau-Kleffner syndrome who underwent MST targeting the posterior temporal lobe versus those who did not undergo intervention [30]. Also, late seizure recurrence in patients with initially favorable outcomes after MST have been described [95]. Given these mixed experiences, and a lack of controlled data, a large prospective investigation of MST in treating patients with eloquent seizure foci would be valuable.
Corpus callosotomy (CC)
Callosotomy, a partial or complete division of the corpus callosum, was introduced as a palliative surgical treatment for epilepsy van Wagenen and Herren in 1940 [124]. By destroying the major commissural connection between the two hemispheres, callosotomy prevents contralateral spread of focal seizure activity, and thus averts ictal loss of consciousness and drop attacks (tonic and atonic seizures) [124,5]. While many investigators have suggested that only anterior callosotomy is necessary to achieve clinical benefit, others advocate for a complete callosotomy [87]. One group has recently proposed performing a callosotomy using an endoscopic approach [114].
While disconnection or “split-brain” syndromes have been a classic fear with callosotomy, postoperative debilitation is rare [76,88]. Complete seizure freedom is also rare after this procedure, but a decrease in the frequency of incapacitating seizures is typically reported [115,86,25,69]. In one large pediatric patient series, a complete arrest of drop attacks was reported in 67% of children after partial anterior callosotomy, and in 91% of those receiving complete callosotomy [113]. However, long-term results suggest that only 35% of callosotomy patients remain free of drop attacks five years after surgery, although the frequency of these seizures remains reduced in most patients [119,98]. Callosotomy utilization has decreased since the introduction of VNS, which also helps prevent tonic and atonic seizures, though there is disagreement regarding which intervention has the best efficacy/risk profile for this purpose [139,26,1,108,138]. One recent systematic review suggests that callosotomy may be more effective than VNS in reducing atonic seizure frequency [105]. It has also been proposed that both therapies may be employed together in certain patients with particularly debilitating drop attacks [64]. In summary, although the clinical benefits of callosotomy are more modest than resective epilepsy surgery, it remains a useful tool in select patients with incapacitating drop attacks, particularly as an alternative to or after failure of VNS. Callosotomy outcomes in patients who have already failed open resection are lacking, and this topic is worthy of further study.
Discussion and Conclusions
Compared to individuals without epilepsy, individuals with drug-resistant epilepsy suffer from increased morbidity and a higher rate of mortality [116,121,129], as well as neuropsychological and neurocognitive deficits and diminished quality of life [39,73,32]. Thus, continued improvements in surgical treatments for epilepsy are critically needed. Surgical resection remains the gold standard treatment for intractable epilepsy, but it is typically not performed in patients with poorly localized seizures, multifocal EZs, or an EZ which co-localizes with eloquent cortex. Furthermore, some patients harbor significant risk factors for an open surgical procedure, or are averse to it. While resection for epilepsy is safe, with approximately 2% significant morbidity and 0.24% surgical mortality [121,116,129], further improvements in the safety profile of invasive epilepsy treatments are needed. For these reasons, non-resective procedures for intractable seizures are becoming increasingly important in the treatment of this disorder.
Seizure outcomes and advantages/disadvantages of non-resective procedures discussed in the present review are summarized in Table 1 and Table 2, respectively. Importantly, while ablative treatment options such as SLA and SRS may replace resection in certain cases, current neurostimulation and disconnection procedures remain palliative and should not be considered replacements for resection. Seizure freedom is the single most important predictor of quality of life in epilepsy, but complete seizure freedom is dramatically less common with neuromodulation or disconnection procedures compared to open resection. While seizure freedom rates with ablative techniques also remain inferior to resection, continued improvement in our understanding and application of these technologies will hopefully lead to progressive improvements in seizure outcomes with minimally invasive interventions for epilepsy.
Table 1.
Summary of seizure outcomes after non-resective surgery for epilepsy.
| Treatment | Seizure outcomes* | Follow-up (months) | Example references |
|---|---|---|---|
| A) Ablative Procedures | |||
| Stereotactic Laser Ablation (SLA) | 36–54% seizure free | 12–14 | 31, 77, 84, 133 |
| Stereotactic Radiosurgery (SRS) | 69–77% seizure free | 24–36 | 7,17, 137 |
| B) Neurostimulation Procedures | |||
| Vagus Nerve Stimulation (VNS) | 51–63% reduced frequency; | 12–24 | 3, 28, 43, 52, 110 |
| 51–57% response rate | |||
| Deep Brain Stimulation (DBS) | 41–69% reduced frequency; | 12–60 | 54,109 |
| 43–68% response rate | |||
| Responsive Neurostimulation (RNS) | 44–66% reduced frequency; | 12–72 | 12,71 |
| 44–59% response rate | |||
| C) Disconnection Procedures | |||
| Multiple Subpial Transections (MST) | 33–71% (near) seizure free; higher when combined with resection | > 12 | 11, 13, 100, 117 |
| Corpus Callosotomy (CC) | 35–91% reduced frequency of drop attacks | > 12 | 98, 105, 113, 119 |
Outcome measures differ between interventions, as seizure freedom is the primary treatment goal in ablative procedures (A), while reduction of seizure frequency is more often the goal with palliative neurostimulation procedures (B). Many MST outcomes have reported “near” seizure freedom rates, while the primary goal of callosotomy is reduction of drop attacks (C).
Table 2.
Advantages and disadvantages of non-resective epilepsy procedures.
| Treatment | Advantages | Disadvantages |
|---|---|---|
| A) Ablative Procedures | ||
| Stereotactic Laser Ablation (SLA) | May be curative; less invasive than resection | Appears less efficacious than resection |
| Stereotactic Radiosurgery (SRS) | May be curative; less invasive than open surgery | Delayed benefit of 1–2 years |
| B) Neurostimulation Procedures | ||
| Vagus Nerve Stimulation (VNS) | No intracranial surgery; EZ localization not necessary | Palliative; complete seizure freedom is rare; implanted hardware |
| Deep Brain Stimulation (DBS) | EZ localization not necessary | Palliative; requires intracranial hardware; not closed loop |
| Responsive Neurostimulation (RNS) | Can treat eloquent EZ; closed loop | Palliative; requires intracranial hardware; EZ localization is necessary |
| C) Disconnection Procedures | ||
| Multiple Subpial Transections (MST) | Can treat eloquent EZ; no implanted hardware | Efficaciousness remains unclear; risk of neurological deficit |
| Corpus Callosotomy (CC) | Relatively efficacious for atonic seizures; no implanted hardware | Palliative; only useful for patients with atonic seizures |
EZ: epileptogenic zone.
Cost effectiveness research has suggested that resective epilepsy surgery is more economically effective than continued medical therapy in both children and adults [14,111,128], but few cost effectiveness studies have examined non-resective epilepsy procedures. Ben-Menachem et al. performed a retrospective analysis of 43 patients receiving VNS for epilepsy, and concluded that VNS results in annual reduction of approximately 3,000 dollars (in 2002 U.S. currency) in unplanned hospital costs per patient [9]. Helmers and colleagues performed a retrospective analysis of U.S. Medicaid data, and concluded that VNS is associated with cost savings and decreased use of resources in children with intractable epilepsy, as compared to medical therapy alone [72]. However, cost analyses of most other non-resective epilepsy procedures, including ablation and stimulation techniques, have not yet been reported.
One important goal in the development of novel therapies for intractable epilepsy is to increase the number of patients who are candidates for or amenable to treatment. Less than 5% of patients with drug-resistant epilepsy – which is defined after the failure of two or more anti-epileptic drug trials – enter remission each year with continued medical therapy alone [21,58,121,81,80,130]. Therefore, the American Academy of Neurology, the American Association of Neurological Surgeons, the National Association of Epilepsy Centers, and the International League Against Epilepsy all agree that individuals who have failed two or more anti-epileptic medications should be referred to a comprehensive epilepsy center for surgical evaluation [24,35,82]. Unfortunately, the utilization of resection for epilepsy remains dramatically under-utilized, with only a minority of potentially eligible candidates receiving surgical treatment each year [47,68,48,36,33,38]. In the near future, it will be important to study whether novel non-resective surgical options for epilepsy will increase the number of patients who receive treatment – or at the very least stimulate more referrals to centers where patients can receive a comprehensive evaluation. Such referrals are critical, given the significant deleterious effects of recurrent seizures on quality of life and survival in patients with epilepsy.
Figure 3. The responsive neurostimulation device (RNS).
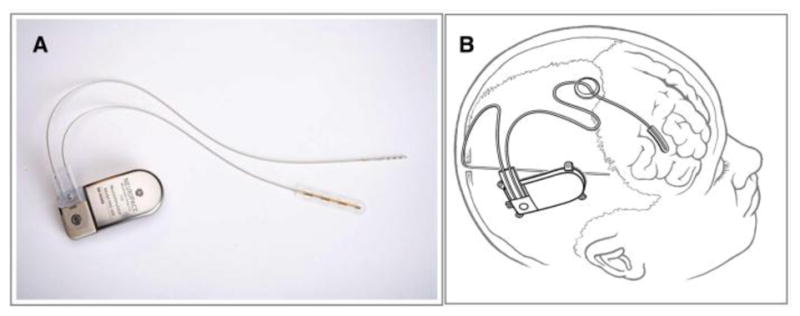
A) Shown is a NeuroPace RNS device configured for stimulation of one four-contact depth electrode and one four-contact strip electrode. B) Artistic depiction of implanted RNS device, including a depth electrode and a cortical strip electrode. Images provided courtesy of NeuroPace.
Footnotes
Disclosures: The author has nothing to disclose.
References
- 1.Abd-El-Barr MM, Joseph JR, Schultz R, Edmonds JL, Wilfong AA, Yoshor D. Vagus nerve stimulation for drop attacks in a pediatric population. Epilepsy & behavior : E&B. 2010;19:394–399. doi: 10.1016/j.yebeh.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Apuzzo ML, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2008;62(Suppl 2):506–513. doi: 10.1227/01.neu.0000316253.54651.5e. [DOI] [PubMed] [Google Scholar]
- 3.Amar AP, Heck CN, Levy ML, Smith T, DeGiorgio CM, Oviedo S, Apuzzo ML. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–1276. doi: 10.1097/00006123-199812000-00001. discussion 1276–1280. [DOI] [PubMed] [Google Scholar]
- 4.Andrade DM, Zumsteg D, Hamani C, Hodaie M, Sarkissian S, Lozano AM, Wennberg RA. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66:1571–1573. doi: 10.1212/01.wnl.0000206364.19772.39. [DOI] [PubMed] [Google Scholar]
- 5.Asadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy & behavior : E&B. 2008;13:271–278. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma H. Recent developments in the study of the columnar arrangement of neurons within the motor cortex. Physiological reviews. 1975;55:143–156. doi: 10.1152/physrev.1975.55.2.143. [DOI] [PubMed] [Google Scholar]
- 7.Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, Larson DA, Dillon W, Verhey L, Garcia P, Steiner L, Heck C, Kondziolka D, Beach R, Olivero W, Witt TC, Salanova V, Goodman R. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Annals of neurology. 2009;65:167–175. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Menachem E. Neurostimulation-past, present, and beyond. Epilepsy currents /American Epilepsy Society. 2012;12:188–191. doi: 10.5698/1535-7511-12.5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Menachem E, Hellstrom K, Verstappen D. Analysis of direct hospital costs before and 18 months after treatment with vagus nerve stimulation therapy in 43 patients. Neurology. 2002;59:S44–47. doi: 10.1212/wnl.59.6_suppl_4.s44. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, Tarver WB, Wernicke JF. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 11.Benifla M, Otsubo H, Ochi A, Snead OC, 3rd, Rutka JT. Multiple subpial transections in pediatric epilepsy: indications and outcomes. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006;22:992–998. doi: 10.1007/s00381-006-0122-7. [DOI] [PubMed] [Google Scholar]
- 12.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O'Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blount JP, Langburt W, Otsubo H, Chitoku S, Ochi A, Weiss S, Snead OC, Rutka JT. Multiple subpial transections in the treatment of pediatric epilepsy. Journal of neurosurgery. 2004;100:118–124. doi: 10.3171/ped.2004.100.2.0118. [DOI] [PubMed] [Google Scholar]
- 14.Bowen JM, Snead OC, Chandra K, Blackhouse G, Goeree R. Epilepsy care in ontario: an economic analysis of increasing access to epilepsy surgery. Ontario health technology assessment series. 2012;12:1–41. [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley R, Estronza-Ojeda S, Ojemann JG. Laser Ablation in Pediatric Epilepsy. Neurosurgery clinics of North America. 2016;27:69–78. doi: 10.1016/j.nec.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Chang EF, Englot DJ, Vadera S. Minimally invasive surgical approaches for temporal lobe epilepsy. Epilepsy & behavior : E&B. 2015;47:24–33. doi: 10.1016/j.yebeh.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, Broshek DK, Barbaro NM. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–172. doi: 10.1212/WNL.0b013e3181c9185d. 74/2/165 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, Broshek DK, Barbaro NM Epilepsy Radiosurgery Study G. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–172. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chase MH, Nakamura Y, Clemente CD, Sterman MB. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain research. 1967;5:236–249. doi: 10.1016/0006-8993(67)90089-3. 0006-8993(67)90089-3 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Chen N, Gao Y, Yan N, Liu C, Zhang JG, Xing WM, Kong DM, Meng FG. High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience. 2014;256:370–378. doi: 10.1016/j.neuroscience.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 21.Choi H, Heiman GA, Munger Clary H, Etienne M, Resor SR, Hauser WA. Seizure remission in adults with long-standing intractable epilepsy: an extended follow-up. Epilepsy research. 2011;93:115–119. doi: 10.1016/j.eplepsyres.2010.11.005. S0920-1211(10)00330-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Sell RL, Lenert L, Muennig P, Goodman RR, Gilliam FG, Wong JB. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. Jama. 2008;300:2497–2505. doi: 10.1001/jama.2008.771. 300/21/2497 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Cooke PM, Snider RS. Some cerebellar influences on electrically induced cerebral seizures. Epilepsia. 1955;4:19–28. doi: 10.1111/j.1528-1157.1955.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 24.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, Guerrini R, Mathern GW International League against Epilepsy SfPES, Commissions of N, Paediatrics. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–959. doi: 10.1111/j.1528-1167.2006.00569.x. EPI569 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Cukiert A, Burattini JA, Mariani PP, Camara RB, Seda L, Baldauf CM, Argentoni M, Baise-Zung C, Forster CR, Mello VA. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia. 2006;47:371–374. doi: 10.1111/j.1528-1167.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 26.Cukiert A, Cukiert CM, Burattini JA, Lima AM, Forster CR, Baise C, Argentoni-Baldochi M. Long-term outcome after callosotomy or vagus nerve stimulation in consecutive prospective cohorts of children with Lennox-Gastaut or Lennox-like syndrome and non-specific MRI findings. Seizure : the journal of the British Epilepsy Association. 2013 doi: 10.1016/j.seizure.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Cyberonics I. [Accessed December 20, 2012 2012];Cyberonics Announces 100,000th Patient Implant of VNS Therapy®. 2012 [Google Scholar]
- 28.DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, Murphy J, Olejniczak P, Shih J, Arrambide S, Soss J. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology. 2005;65:317–319. doi: 10.1212/01.wnl.0000168899.11598.00. 65/2/317 [pii] [DOI] [PubMed] [Google Scholar]
- 29.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collins S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Labar D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 30.Downes M, Greenaway R, Clark M, Helen Cross J, Jolleff N, Harkness W, Kaliakatsos M, Boyd S, White S, Neville BG. Outcome following multiple subpial transection in Landau-Kleffner syndrome and related regression. Epilepsia. 2015 doi: 10.1111/epi.13132. [DOI] [PubMed] [Google Scholar]
- 31.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, Saindane AM, Phatak V, Ivanisevic M, Millis S, Helmers SL, Miller JW, Meador KJ, Gross RE. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–113. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel J., Jr Surgical treatment for epilepsy: too little, too late? Jama. 2008;300:2548–2550. doi: 10.1001/jama.2008.756. 300/21/2548 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Engel J., Jr Why is there still doubt to cut it out? Epilepsy currents /American Epilepsy Society. 2013;13:198–204. doi: 10.5698/1535-7597-13.5.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. Jama. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 36.Englot DJ. Epilepsy surgery trends in the United States: Differences between children and adults. Epilepsia. 2015;56:1321. doi: 10.1111/epi.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Englot DJ. Failed epilepsy surgery: It is not too late. Epilepsy research. 2015;113:151–152. doi: 10.1016/j.eplepsyres.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Englot DJ. The persistent under-utilization of epilepsy surgery. Epilepsy research. 2015;118:68–69. doi: 10.1016/j.eplepsyres.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Progress in brain research. 2009;177:147–170. doi: 10.1016/S0079-6123(09)17711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. Journal of neurosurgery Pediatrics. 2013;12:126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 41.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurgical review. 2014;37:389–404. doi: 10.1007/s10143-014-0527-9. discussion 404-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurgery clinics of North America. 2011;22:443–448. v. doi: 10.1016/j.nec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. Journal of neurosurgery. 2011;115:1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 44.Englot DJ, Lee AT, Tsai C, Halabi C, Barbaro NM, Auguste KI, Garcia PA, Chang EF. Seizure types and frequency in patients who "fail" temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73:838–844. doi: 10.1227/NEU.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 45.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78:1200–1206. doi: 10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Englot DJ, Ouyang D, Wang DD, Rolston JD, Garcia PA, Chang EF. Relationship between hospital surgical volume, lobectomy rates, and adverse perioperative events at US epilepsy centers. Journal of neurosurgery. 2013;118:169–174. doi: 10.3171/2012.9.JNS12776. [DOI] [PubMed] [Google Scholar]
- 49.Englot DJ, Raygor KP, Molinaro AM, Garcia PA, Knowlton RC, Auguste KI, Chang EF. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648–656. doi: 10.1227/NEU.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Englot DJ, Rolston JD, Wang DD, Hassnain KH, Gordon CM, Chang EF. Efficacy of vagus nerve stimulation in posttraumatic versus nontraumatic epilepsy. Journal of neurosurgery. 2012;117:970–977. doi: 10.3171/2012.8.JNS122. [DOI] [PubMed] [Google Scholar]
- 51.Englot DJ, Rolston JD, Wang DD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after temporal lobectomy in pediatric patients. Journal of neurosurgery Pediatrics. 2013;12:134–141. doi: 10.3171/2013.5.PEDS12526. [DOI] [PubMed] [Google Scholar]
- 52.Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016 doi: 10.1227/NEU.0000000000001165. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FDA. [Accessed March 30 2013];Device Approvals and Clearances. 2013 [Google Scholar]
- 54.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Group SS. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 55.Fisher RS. Therapeutic devices for epilepsy. Annals of neurology. 2012;71:157–168. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP, Gordon B, Schwerdt P, Rise M. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 57.Fridley J, Thomas JG, Navarro JC, Yoshor D. Brain stimulation for the treatment of epilepsy. Neurosurgical focus. 2012;32:E13. doi: 10.3171/2012.1.FOCUS11334. [DOI] [PubMed] [Google Scholar]
- 58.Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48:1303–1307. doi: 10.1111/j.1528-1167.2007.01136.x. EPI1136 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Gianaris T, Witt T, Barbaro NM. Radiosurgery for Medial Temporal Lobe Epilepsy Resulting from Mesial Temporal Sclerosis. Neurosurgery clinics of North America. 2016;27:79–82. doi: 10.1016/j.nec.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Martinez J, Vadera S, Mullin J, Enatsu R, Alexopoulos AV, Patwardhan R, Bingaman W, Najm I. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: operative technique. Neurosurgery. 2014;10(Suppl 2):167–172. doi: 10.1227/NEU.0000000000000286. discussion 172–163. [DOI] [PubMed] [Google Scholar]
- 61.Gross RE, Willie JT, Drane DL. The Role of Stereotactic Laser Amygdalohippocampotomy in Mesial Temporal Lobe Epilepsy. Neurosurgery clinics of North America. 2016;27:37–50. doi: 10.1016/j.nec.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guenot M, Isnard J, Catenoix H, Mauguiere F, Sindou M. SEEG-guided RF-thermocoagulation of epileptic foci: a therapeutic alternative for drug-resistant non-operable partial epilepsies. Advances and technical standards in neurosurgery. 2011;36:61–78. doi: 10.1007/978-3-7091-0179-7_4. [DOI] [PubMed] [Google Scholar]
- 63.Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguiere F, Sindou M. SEEG-guided RF thermocoagulation of epileptic foci: feasibility, safety, and preliminary results. Epilepsia. 2004;45:1368–1374. doi: 10.1111/j.0013-9580.2004.17704.x. [DOI] [PubMed] [Google Scholar]
- 64.Guillamon E, Miro J, Gutierrez A, Conde R, Falip M, Jaraba S, Plans G, Garces M, Villanueva V. Combination of corpus callosotomy and vagus nerve stimulation in the treatment of refractory epilepsy. European neurology. 2014;71:65–74. doi: 10.1159/000353979. [DOI] [PubMed] [Google Scholar]
- 65.Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 2015;56:114–124. doi: 10.1111/epi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han CL, Hu W, Stead M, Zhang T, Zhang JG, Worrell GA, Meng FG. Electrical stimulation of hippocampus for the treatment of refractory temporal lobe epilepsy. Brain research bulletin. 2014;109:13–21. doi: 10.1016/j.brainresbull.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL, 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 68.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75:699–704. doi: 10.1212/WNL.0b013e3181eee457. 75/8/699 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanson RR, Risinger M, Maxwell R. The ictal EEG as a predictive factor for outcome following corpus callosum section in adults. Epilepsy research. 2002;49:89–97. doi: 10.1016/s0920-1211(01)00328-x. [DOI] [PubMed] [Google Scholar]
- 70.Hawasli AH, Bandt SK, Hogan RE, Werner N, Leuthardt EC. Laser ablation as treatment strategy for medically refractory dominant insular epilepsy: therapeutic and functional considerations. Stereotactic and functional neurosurgery. 2014;92:397–404. doi: 10.1159/000366001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP, Skidmore C, Van Ness PC, Bergey GK, Park YD, Miller I, Geller E, Rutecki PA, Zimmerman R, Spencer DC, Goldman A, Edwards JC, Leiphart JW, Wharen RE, Fessler J, Fountain NB, Worrell GA, Gross RE, Eisenschenk S, Duckrow RB, Hirsch LJ, Bazil C, O'Donovan CA, Sun FT, Courtney TA, Seale CG, Morrell MJ. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helmers SL, Duh MS, Guerin A, Sarda SP, Samuelson TM, Bunker MT, Olin BD, Jackson SD, Faught E. Clinical outcomes, quality of life, and costs associated with implantation of vagus nerve stimulation therapy in pediatric patients with drug-resistant epilepsy. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2012;16:449–458. doi: 10.1016/j.ejpn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl 2):96–98. doi: 10.1111/j.1528-1167.2006.00702.x. EPI702 [pii] [DOI] [PubMed] [Google Scholar]
- 74.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 75.Homma J, Kameyama S, Masuda H, Ueno T, Fujimoto A, Oishi M, Fukuda M. Stereotactic radiofrequency thermocoagulation for hypothalamic hamartoma with intractable gelastic seizures. Epilepsy research. 2007;76:15–21. doi: 10.1016/j.eplepsyres.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Jea A, Vachhrajani S, Widjaja E, Nilsson D, Raybaud C, Shroff M, Rutka JT. Corpus callosotomy in children and the disconnection syndromes: a review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24:685–692. doi: 10.1007/s00381-008-0626-4. [DOI] [PubMed] [Google Scholar]
- 77.Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, Skidmore C, Mintzer S, Sharan AD, Sperling MR. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2015 doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 78.King-Stephens D, Mirro E, Weber PB, Laxer KD, Van Ness PC, Salanova V, Spencer DC, Heck CN, Goldman A, Jobst B, Shields DC, Bergey GK, Eisenschenk S, Worrell GA, Rossi MA, Gross RE, Cole AJ, Sperling MR, Nair DR, Gwinn RP, Park YD, Rutecki PA, Fountain NB, Wharen RE, Hirsch LJ, Miller IO, Barkley GL, Edwards JC, Geller EB, Berg MJ, Sadler TL, Sun FT, Morrell MJ. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56:959–967. doi: 10.1111/epi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koo B. EEG changes with vagus nerve stimulation. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2001;18:434–441. doi: 10.1097/00004691-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 81.Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(Suppl 8):57–62. doi: 10.1111/j.1528-1167.2009.02237.x. EPI2237 [pii] [DOI] [PubMed] [Google Scholar]
- 82.Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ National Association of Epilepsy C. Essential services, personnel, and facilities in specialized epilepsy centers--revised 2010 guidelines. Epilepsia. 2010;51:2322–2333. doi: 10.1111/j.1528-1167.2010.02648.x. EPI2648 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11:508–526. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis EC, Weil AG, Duchowny M, Bhatia S, Ragheb J, Miller I. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 2015;56:1590–1598. doi: 10.1111/epi.13106. [DOI] [PubMed] [Google Scholar]
- 85.Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia. 2015;56:1836–1844. doi: 10.1111/epi.13191. [DOI] [PubMed] [Google Scholar]
- 86.Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42:67–71. doi: 10.1046/j.1528-1157.2001.081422.x. [DOI] [PubMed] [Google Scholar]
- 87.Malmgren K, Rydenhag B, Hallbook T. Reappraisal of corpus callosotomy. Curr Opin Neurol. 2015;28:175–181. doi: 10.1097/WCO.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 88.Mamelak AN, Barbaro NM, Walker JA, Laxer KD. Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. Journal of neurosurgery. 1993;79:688–695. doi: 10.3171/jns.1993.79.5.0688. [DOI] [PubMed] [Google Scholar]
- 89.McCracken DJ, Willie JT, Fernald BA, Saindane AM, Drane DL, Barrow DL, Gross RE. Magnetic Resonance Thermometry-Guided Stereotactic Laser Ablation of Cavernous Malformations in Drug-Resistant Epilepsy: Imaging and Clinical Results. Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meador KJ, Kapur R, Loring DW, Kanner AM, Morrell MJ Investigators RNSSPT. Quality of life and mood in patients with medically intractable epilepsy treated with targeted responsive neurostimulation. Epilepsy & behavior : E&B. 2015;45:242–247. doi: 10.1016/j.yebeh.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. Journal of neurosurgery. 1989;70:231–239. doi: 10.3171/jns.1989.70.2.0231. [DOI] [PubMed] [Google Scholar]
- 92.Morrell MJ Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 93.Moruzzi G. Effects at different frequencies of cerebellar stimulation upon postural tonus and myotatic reflexes. Electroencephalography and clinical neurophysiology. 1950;2:463–469. doi: 10.1016/0013-4694(50)90083-6. [DOI] [PubMed] [Google Scholar]
- 94.Ntsambi-Eba G, Vaz G, Docquier MA, van Rijckevorsel K, Raftopoulos C. Patients with refractory epilepsy treated using a modified multiple subpial transection technique. Neurosurgery. 2013;72:890–897. doi: 10.1227/NEU.0b013e31828ba750. discussion 897–898. [DOI] [PubMed] [Google Scholar]
- 95.Orbach D, Romanelli P, Devinsky O, Doyle W. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001;42:1316–1319. doi: 10.1111/j.1528-1167.2001.45300.x. [DOI] [PubMed] [Google Scholar]
- 96.Panebianco M, Rigby A, Weston J, Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev. 2015;4:CD002896. doi: 10.1002/14651858.CD002896.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia. 1999;40:1408–1416. doi: 10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 98.Passamonti C, Zamponi N, Foschi N, Trignani R, Luzi M, Cesaroni E, Provinciali L, Scerrati M. Long-term seizure and behavioral outcomes after corpus callosotomy. Epilepsy & behavior : E&B. 2014;41:23–29. doi: 10.1016/j.yebeh.2014.08.130. [DOI] [PubMed] [Google Scholar]
- 99.Patil AA, Andrews R. Long term follow-up after multiple hippocampal transection (MHT) Seizure : the journal of the British Epilepsy Association. 2013;22:731–734. doi: 10.1016/j.seizure.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 100.Polkey CE. Multiple subpial transection: a clinical assessment. Int Rev Neurobiol. 2001;45:547–569. doi: 10.1016/s0074-7742(01)45028-8. [DOI] [PubMed] [Google Scholar]
- 101.Quigg M, Broshek DK, Barbaro NM, Ward MM, Laxer KD, Yan G, Lamborn K Radiosurgery Epilepsy Study G. Neuropsychological outcomes after Gamma Knife radiosurgery for mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2011;52:909–916. doi: 10.1111/j.1528-1167.2011.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Regis J, Bartolomei F, Hayashi M, Roberts D, Chauvel P, Peragut JC. The role of gamma knife surgery in the treatment of severe epilepsies. Epileptic disorders : international epilepsy journal with videotape. 2000;2:113–122. [PubMed] [Google Scholar]
- 103.Regis J, Bartolomei F, Rey M, Hayashi M, Chauvel P, Peragut JC. Gamma knife surgery for mesial temporal lobe epilepsy. Journal of neurosurgery. 2000;93(Suppl 3):141–146. doi: 10.3171/jns.2000.93.supplement3.0141. [DOI] [PubMed] [Google Scholar]
- 104.Regis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schrottner O, Pendl G. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504–515. doi: 10.1111/j.0013-9580.2004.07903.x. [DOI] [PubMed] [Google Scholar]
- 105.Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: A systematic review. Epilepsy & behavior : E&B. 2015;51:13–17. doi: 10.1016/j.yebeh.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rolston JD, Englot DJ, Wang DD, Shih T, Chang EF. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurgical focus. 2012;32:E14. doi: 10.3171/2012.1.FOCUS11335. [DOI] [PubMed] [Google Scholar]
- 107.Rolston JD, Quigg M, Barbaro NM. Gamma knife radiosurgery for mesial temporal lobe epilepsy. Epilepsy research and treatment. 2011;2011:840616. doi: 10.1155/2011/840616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenfeld WE, Roberts DW. Tonic and atonic seizures: what's next--VNS or callosotomy? Epilepsia. 2009;50(Suppl 8):25–30. doi: 10.1111/j.1528-1167.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 109.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Troster AI, Irwin CP, Lambrecht K, Graves N, Fisher R, Group SS. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scherrmann J, Hoppe C, Kral T, Schramm J, Elger CE. Vagus nerve stimulation: clinical experience in a large patient series. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2001;18:408–414. doi: 10.1097/00004691-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 111.Schiltz NK, Kaiboriboon K, Koroukian SM, Singer ME, Love TE. Long-term reduction of health care costs and utilization after epilepsy surgery. Epilepsia. 2015 doi: 10.1111/epi.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmitt FC, Voges J, Buentjen L, Woermann F, Pannek HW, Skalej M, Heinze HJ, Ebner A. Radiofrequency lesioning for epileptogenic periventricular nodular heterotopia: a rational approach. Epilepsia. 2011;52:e101–105. doi: 10.1111/j.1528-1167.2011.03116.x. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46(Suppl 1):30–31. doi: 10.1111/j.0013-9580.2005.461009.x. [DOI] [PubMed] [Google Scholar]
- 114.Sood S, Marupudi NI, Asano E, Haridas A, Ham SD. Endoscopic corpus callosotomy and hemispherotomy. Journal of neurosurgery Pediatrics. 2015:1–6. doi: 10.3171/2015.5.PEDS1531. [DOI] [PubMed] [Google Scholar]
- 115.Spencer DD, Spencer SS. Corpus callosotomy in the treatment of medically intractable secondarily generalized seizures of children. Cleve Clin J Med. 1989;56(Suppl Pt 1):S69–78. doi: 10.3949/ccjm.56.s1.69. discussion S79–83. [DOI] [PubMed] [Google Scholar]
- 116.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. The Lancet Neurology. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. S1474-4422(08)70109-1 [pii] [DOI] [PubMed] [Google Scholar]
- 117.Spencer SS, Schramm J, Wyler A, O'Connor M, Orbach D, Krauss G, Sperling M, Devinsky O, Elger C, Lesser R, Mulligan L, Westerveld M. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141–145. doi: 10.1046/j.1528-1157.2002.28101.x. [DOI] [PubMed] [Google Scholar]
- 118.Sun XR, Patel NV, Danish SF. Tissue Ablation Dynamics During Magnetic Resonance-Guided, Laser-Induced Thermal Therapy. Neurosurgery. 2015;77:51–58. doi: 10.1227/NEU.0000000000000732. discussion 58. [DOI] [PubMed] [Google Scholar]
- 119.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain : a journal of neurology. 2005;128:1188–1198. doi: 10.1093/brain/awh449. awh449 [pii] [DOI] [PubMed] [Google Scholar]
- 120.Tellez-Zenteno JF, Wiebe S. Hippocampal stimulation in the treatment of epilepsy. Neurosurgery clinics of North America. 2011;22:465–475. vi. doi: 10.1016/j.nec.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 121.Thom M, Mathern GW, Cross JH, Bertram EH. Mesial temporal lobe epilepsy: How do we improve surgical outcome? Annals of neurology. 2010;68:424–434. doi: 10.1002/ana.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tyrand R, Seeck M, Pollo C, Boex C. Effects of amygdala-hippocampal stimulation on synchronization. Epilepsy research. 2014;108:327–330. doi: 10.1016/j.eplepsyres.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 123.Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, Torres N, Pastor J, Selway R, Sola RG, Alarcon G. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54:1823–1833. doi: 10.1111/epi.12352. [DOI] [PubMed] [Google Scholar]
- 124.Van Wagenen W, Herren R. Surgical division of commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Arch NeurPsych. 1940;44:740–759. [Google Scholar]
- 125.Velasco AL, Velasco F, Jimenez F, Velasco M, Castro G, Carrillo-Ruiz JD, Fanghanel G, Boleaga B. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 126.Vesper J, Steinhoff B, Rona S, Wille C, Bilic S, Nikkhah G, Ostertag C. Chronic high-frequency deep brain stimulation of the STN/SNr for progressive myoclonic epilepsy. Epilepsia. 2007;48:1984–1989. doi: 10.1111/j.1528-1167.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- 127.Waseem H, Osborn KE, Schoenberg MR, Kelley V, Bozorg A, Cabello D, Benbadis SR, Vale FL. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy & behavior : E&B. 2015;51:152–157. doi: 10.1016/j.yebeh.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 128.Widjaja E, Li B, Schinkel CD, Puchalski Ritchie L, Weaver J, Snead OC, Rutka JT, Coyte PC. Cost-effectiveness of pediatric epilepsy surgery compared to medical treatment in children with intractable epilepsy. Epilepsy research. 2011;94:61–68. doi: 10.1016/j.eplepsyres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 129.Wiebe S. Effectiveness and safety of epilepsy surgery: what is the evidence? CNS Spectr. 2004;9:120–122. 126–132. doi: 10.1017/s1092852900008488. [DOI] [PubMed] [Google Scholar]
- 130.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 131.Wilfong AA, Curry DJ. Hypothalamic hamartomas: optimal approach to clinical evaluation and diagnosis. Epilepsia. 2013;54(Suppl 9):109–114. doi: 10.1111/epi.12454. [DOI] [PubMed] [Google Scholar]
- 132.Wille C, Steinhoff BJ, Altenmuller DM, Staack AM, Bilic S, Nikkhah G, Vesper J. Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood--report of five cases. Epilepsia. 2011;52:489–496. doi: 10.1111/j.1528-1167.2010.02884.x. [DOI] [PubMed] [Google Scholar]
- 133.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, Brat DJ, Helmers SL, Saindane A, Nour SG, Gross RE. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–584. doi: 10.1227/NEU.0000000000000343. discussion 584–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu C, Boorman DW, Gorniak RJ, Farrell CJ, Evans JJ, Sharan AD. The effects of anatomic variations on stereotactic laser amygdalohippocampectomy and a proposed protocol for trajectory planning. Neurosurgery. 2015;11(Suppl 2):345–356. doi: 10.1227/NEU.0000000000000767. discussion 356–347. [DOI] [PubMed] [Google Scholar]
- 135.Wyckhuys T, Raedt R, Vonck K, Wadman W, Boon P. Comparison of hippocampal Deep Brain Stimulation with high (130Hz) and low frequency (5Hz) on afterdischarges in kindled rats. Epilepsy research. 2010;88:239–246. doi: 10.1016/j.eplepsyres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 136.Wyler AR, Wilkus RJ, Rostad SW, Vossler DG. Multiple subpial transections for partial seizures in sensorimotor cortex. Neurosurgery. 1995;37:1122–1127. doi: 10.1227/00006123-199512000-00012. discussion 1127–1128. [DOI] [PubMed] [Google Scholar]
- 137.Yen DJ, Chung WY, Shih YH, Chen C, Lirng JF, Yiu CH, Yu HY, Su TP, Pan DH. Gamma knife radiosurgery for the treatment of recurrent seizures after incomplete anterior temporal lobectomy. Seizure : the journal of the British Epilepsy Association. 2009;18:511–514. doi: 10.1016/j.seizure.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 138.You SJ, Kang HC, Ko TS, Kim HD, Yum MS, Hwang YS, Lee JK, Kim DS, Park SK. Comparison of corpus callosotomy and vagus nerve stimulation in children with Lennox-Gastaut syndrome. Brain & development. 2008;30:195–199. doi: 10.1016/j.braindev.2007.07.013. S0387-7604(07)00185-4 [pii] [DOI] [PubMed] [Google Scholar]
- 139.Zamponi N, Passamonti C, Cesaroni E, Trignani R, Rychlicki F. Effectiveness of vagal nerve stimulation (VNS) in patients with drop-attacks and different epileptic syndromes. Seizure : the journal of the British Epilepsy Association. 2011;20:468–474. doi: 10.1016/j.seizure.2011.02.011. [DOI] [PubMed] [Google Scholar]