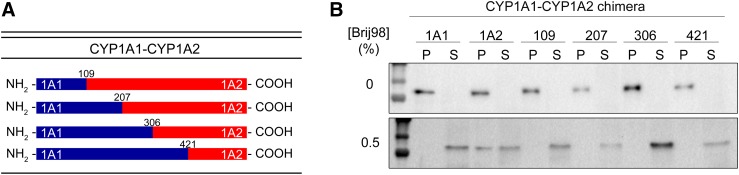
Fig. 2.
Replacement of the NH2-terminal region of CYP1A2 with that of CYP1A1 changes its membrane localization. The microdomain localization CYP1A2 and chimeric proteins where the NH2 terminus was substituted with that of CYP1A1 was measured by expression of the proteins in human embryonic kidney 293T cells and partial membrane solubilization using Brij 98. After Brij treatment, the samples were subjected to centrifugation and the proteins remaining in the membranes were detected in the pellet using PAGE. (A) Schematic of the chimeric proteins generated. (B) Immunoblot of the native CYP1A proteins and the chimeras. Proteins in the pellet remain associated with the membrane, whereas those in the supernatant were solubilized. P, pellet; S, supernatant. This research was originally published in the Journal of Biological Chemistry [Park JW, Reed JR, and Backes WL. The localization of cytochrome P450s CYP1A1 and CYP1A2 into different lipid microdomains is governed by their NH2-terminal and internal protein regions. Journal of Biological Chemistry. (2015) 290, 29449–29460. © American Society for Biochemistry and Molecular Biology].