Abstract
Lipid-lowering treatment with statins is one of the most effective therapeutic strategies in cardiovascular medicine because they reduce cardiovascular risk in both primary and secondary prevention. Despite the well-established links between low-density lipoprotein and cardiovascular risk, the clinical benefit from statin treatment is not fully explained by their lipid-lowering potential. A number of pleiotropic effects of statins have been described over the past decade, and their ability to suppress global oxidative stress is probably one of the most important mechanisms by which they exert their beneficial effects on the cardiovascular system. In this Forum, there are review articles discussing the molecular mechanisms by which statins modify redox signaling in the vasculature and the heart. They exert direct effects on the vascular wall and the myocardium or indirect by targeting the interactions between the cardiovascular system and adipose tissue or circulating cell types. The review articles in this Forum follow a translational approach and link the molecular mechanisms by which statins modify cardiovascular redox signaling with their clinical benefit in the prevention and treatment of cardiovascular diseases. Antioxid. Redox Signal. 20, 1195–1197.
When the first HMG-CoA-reductase inhibitor was developed in the 1970s (i.e., mevastatin), nobody could imagine that this pharmacological intervention would have such an enormous impact in clinical practice across so many different medical fields. This new inhibitor took more than a decade to attract the attention of pharmaceutical industry and led to the development of lovastatin, the first registered statin for clinical use. Since then, statins came to the forefront of cardiovascular pharmacology, and it is now clear that by inhibiting HMG-CoA-reductase and reducing low-density lipoprotein (LDL), they are able to lower cardiovascular risk in both primary and secondary prevention.
Over the past decade, it became clear that statins exert cardiovascular effects beyond lipid lowering, and these were called “pleiotropic effects.” One of the most important aspects of statin treatment is their ability to reduce oxidative stress in various tissue and cell types in the cardiovascular system, and this is believed to be a key mechanism by which they reduce cardiovascular risk. Indeed, oxidative stress is a critical therapeutic target in cardiovascular pharmacology as it is involved in atherogenesis, arrhythmogenesis, and other cardiovascular disorders. In this Forum of ARS, experts in the field of statin pharmacology present the most up-to-date knowledge on the role of statins in the regulation of cardiovascular redox signaling and give their expert opinion on the future potential of statin treatment in cardiovascular science.
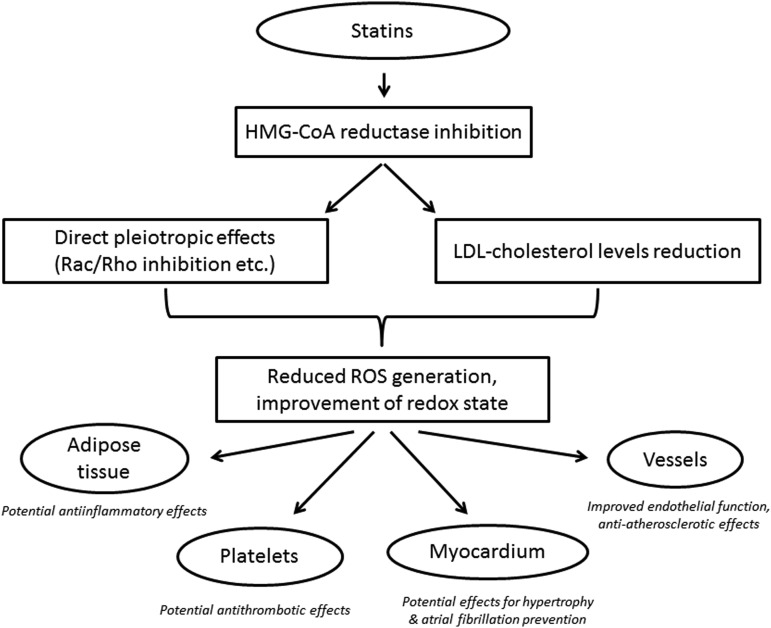
In the first article of this Forum, Margaritis et al. (6) discuss the role of statins as regulators of redox signaling in the human vascular endothelium (Fig. 1). They provide a comprehensive review of the role of oxidative stress in vascular biology and particularly in atherosclerosis. Then, they present the individual sources of reactive oxygen species in the human vascular endothelium (such as NADPH oxidase and uncoupled endothelial nitric oxide synthase [eNOS]), and they discuss their role as potential therapeutic targets in atherosclerosis. Statins appear to modify the activity of NADPH oxidase in the endothelium (by preventing the phosphorylation and membrane translocation of p47phox, p67phox, and Rac1 as well as by affecting the expression of critical NADPH oxidase subunits), whereas they also activate eNOS and improve its enzymatic coupling by restoring the bioavailability of its cofactor tetrahydrobiopterin (BH4).
FIG. 1.
Effects of statins on cardiovascular system.
Importantly, further to the direct pleiotropic effects of statins on the vascular wall, the reduction of LDL-per se is also a critical factor modifying vascular redox state, as discussed by Hermida and Balligand in this Forum of ARS (3). Indeed, LDL suppresses NO bioavailability in the vascular endothelium by affecting eNOS gene expression and enzymatic activity, partly via caveoline-1-dependent mechanisms (3). The molecular mechanisms behind the effects of statins on endothelial redox signaling and the clinical implications of this effect are extensively discussed in this Forum of ARS (3, 6).
Following the first two articles on the effects of statins on endothelial redox state (3, 6), Adam and Laufs (1) follow a different approach and present the role of statins in the regulation of Rac1 GTPase, an established master regulator of cell motility and reactive oxygen species generation (Fig. 1). Since Rac1 consists a critical subunit of NOX1 and NOX2 isoforms of NADPH oxidase and its activation (through binding to GTP and translocation to the cellular membrane) triggers NADPH oxidase, it appears to be an important therapeutic target in many antioxidant strategies. Statins reduce Rac1 activation in the heart and the vascular wall by inhibiting Rac isoprenylation, and this mechanism has been proposed as a major element of the pleiotropic effects of statins in humans (1).
Further to the role of statins in Rac1-mediated regulation of vascular and myocardial redox state, Sawada and Liao (8) focus on the role of HMG-CoA reductase inhibition in the regulation of Rho/ROCK pathway in atherogenesis (Fig. 1). ROCKs play an important role in cellular apoptosis, growth, metabolism, and migration via the control of the actin cytoskeletal assembly and cell contraction (8). Importantly, there is a cross talk between NO and Rho/ROCK signaling in the vascular wall, as endothelial-derived NO diffuses into the adjacent vascular smooth muscle cells (VSMCs) of the blood vessels, activates soluble guanylate cyclase, stimulates the formation of cGMP, and subsequently activates cGMP-dependent protein kinase (cGK). This activation of cGMP/cGK cascade leads to the relaxation of VSMCs and inhibits their proliferation, migration, and fibrotic gene expression. The negative regulation of these VSMC functions by cGMP/cGK pathway has been known to be counteracted by the Rho/ROCK pathway. Importantly, Rho/ROCK adversely affect the stability of eNOS mRNA and they reduce eNOS phosphorylation through inhibition of Akt signaling in human endothelial cells. Statins, by inhibiting mevalonate synthesis, prevent membrane targeting of Rho and the subsequent activation of ROCK. This has been confirmed by clinical trials showing rapid attenuation of ROCK activity in circulating leucocytes after the treatment with statins. However, the extent to which the pleiotropic effects of statins are attributable to Rho/ROCK inhibition remains to be clarified.
Given the critical role of NADPH oxidase in the regulation of both myocardial and vascular redox signaling, its inhibition by statins (e.g., by suppressing Rac activation) could modify oxidative stress in the entire cardiovascular system. Reduction of the NADPH oxidase activity in the heart could prevent atrial fibrosis/remodeling and suppress atrium-derived arrhythmias, such as atrial fibrillation (2). Following this concept, Pinho-Gomes et al. (7) discuss the direct implications of statin treatment in myocardial redox state and physiology. They provide the most up-to-date knowledge on the role of redox signaling in myocardial biology, and they discuss the molecular mechanisms by which statins control oxidative stress in the heart. They also discuss the ability of statins to modify the NADPH oxidase activity and NOS coupling/activation in the heart, and they provide a comprehensive discussion of the molecular mechanisms by which statins could affect arrhythmogenic substrate, hypertrophy, and cardiac remodeling (Fig. 1).
Given the continuous cross talk between the cardiovascular system and other neighboring organs (such as the adipose tissue), statins may exert their cardiovascular effects indirectly, by targeting the signaling between adipose tissue and the heart or the vascular wall. In this Forum of ARS, Lim et al. (4) describes the role of statin treatment in the biology of adipose tissue. Evidence suggests that the cross talk between adipose tissue and the vascular wall results into the regulation of vascular redox state in humans (5). Lipophilic statins appear to have an anti-inflammatory potential within the adipose tissue, therefore they could partly exert their well-established antiatherogenic effects by targeting the human adipose tissue (Fig. 1).
In a similar direction, Violi and Pignatelli (9) support that statins also suppress redox signaling in the circulating platelets. They reduce the NADPH oxidase activity and improve NO bioavailability within the circulating platelets, preventing their activation and adhesion to the endothelium. Therefore, by preventing platelet activation, statins prevent the release of vasoactive mediators that are known to induce endothelial dysfunction (e.g., sCD40-ligand, thromboxane, and others) (9). Violi and Pignatelli (9) support that statins may have antithrombotic effects, providing an additional mechanism by which they exert their well-known antiatherogenic potential (Fig. 1).
In conclusion, the role of statins in the regulation of global oxidative stress via both LDL-dependent and LDL-independent (pleiotropic) mechanisms is now widely accepted. Statins appear to modify redox signaling by acting directly on the heart and the vessels, whereas they also exert indirect effects on cardiovascular physiology by affecting the biology of adipose tissue or circulating cell populations that interact with the cardiovascular system. Investigating the pleiotropic effects of statins is crucial since they offer probably the most effective pharmacological intervention for the prevention or even regression of atherosclerosis and they have a major impact on clinical outcome in both primary and secondary prevention. Better understanding of the molecular mechanisms by which statins affect cardiovascular physiology may help us identify novel therapeutic targets for the prevention and treatment of cardiovascular diseases.
Abbreviations Used
- cGK
cGMP-dependent protein kinase
- eNOS
endothelial nitric oxide synthase
- LDL
low-density lipoprotein
- VSMC
vascular smooth muscle cell
References
- 1.Adam O. and Laufs U. Rac1-mediated effects of HMG-CoA reductase inhibitors (statins) in cardiovascular disease. Antioxid Redox Signal 20: 1238–1250, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM, and Casadei B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol 59: 60–70, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Hermida N. and Balligand J-L. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: the role of statins. Antioxid Redox Signal 20: 1216–1237, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Sakuma I, Quon MJ, and Koh KK. Differential metabolic actions of specific statins: clinical and therapeutic considerations. Antioxid Redox Signal 20: 1286–1299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, and Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127: 2209–2221, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Margaritis M, Channon K, and Antoniades C. Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering. Antioxid Redox Signal 20: 1198–1215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho-Gomes AC, Reilly S, Brandes RP, and Casadei B. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition with statins. Antioxid Redox Signal 20: 1268–1285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada N. and Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal 20: 1251–1267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violi F. and Pignatelli P. Statins as regulators of redox signaling in platelets. Antioxid Redox Signal 20: 1300–1312, 2014. [DOI] [PubMed] [Google Scholar]