Abstract
A total of 57 methicillin-resistant Staphylococcus aureus (MRSA) isolates and 475 methicillin-resistant coagulase-negative staphylococci (MRCoNS) collected from pigs in the Guangdong province of China in 2014 were investigated for the presence of the novel oxazolidinone-phenicol resistance gene optrA. The optrA gene was detected in 6.9% (n = 33) of the MRCoNS, all of which were Staphylococcus sciuri isolates, but in none of the MRSA isolates. Five optrA-carrying methicillin-resistant (MR) S. sciuri isolates also harbored the multiresistance gene cfr. Pulsed-field gel electrophoresis (PFGE) and dru typing of the 33 optrA-carrying MR S. sciuri isolates revealed 25 patterns and 5 sequence types, respectively. S1 nuclease PFGE and Southern blotting confirmed that optrA was located in the chromosomal DNAs of 29 isolates, including 1 cfr-positive isolate. The remaining four isolates harbored a ∼35-kb pWo28-3-like plasmid on which optrA and cfr were located together with other resistance genes, as confirmed by sequence analysis. Six different types of genetic environments (types I to VI) of the chromosome-borne optrA genes were identified; these types had the optrA gene and its transcriptional regulator araC in common. Tn558 was found to be associated with araC-optrA in types II to VI. The optrA gene in types II and III was found in close proximity to the ccr gene complex of the respective staphylococcal cassette chromosome mec element (SCCmec). Since oxazolidinones are last-resort antimicrobial agents for the control of serious infections caused by methicillin-resistant staphylococci in humans, the location of the optrA gene close to the ccr complex is an alarming observation.
INTRODUCTION
Methicillin-resistant coagulase-negative staphylococci (MRCoNS) and methicillin-resistant Staphylococcus aureus (MRSA) play a major role in both health care- and community-associated infections (1, 2). MRCoNS have also been detected in recent years as pathogens in animals (3, 4) as well as in food of animal origin (5) and in non-health care environments (6). Linezolid is one of the few clinically effective drugs for the control of MRSA and MRCoNS infections. Resistance to linezolid in staphylococci is still very rare but has been increasing in recent years (7). Resistance to linezolid in staphylococci is due mainly to mutations in domain V of 23S rRNA or in the genes coding for the ribosomal proteins L3 and L4 (8, 9). However, transferable linezolid resistance due to the cfr gene has been known since the year 2000. The cfr gene was first discovered in a bovine Staphylococcus sciuri strain and was later reported in various Staphylococcus species (10–12). Recently, a cfr homologue, cfr(B), was discovered in Clostridium difficile and Enterococcus faecium (13, 14). Besides resistance to oxazolidinones, the cfr gene also mediates cross-resistance to phenicols, lincosamides, pleuromutilins, and streptogramin A antibiotics (15). Very recently, a novel transferable oxazolidinone resistance gene, optrA, has been identified, mainly in enterococci from humans and animals (16–19). In contrast to cfr, optrA confers cross-resistance only to phenicols and oxazolidinones. This cross-resistance, however, also includes resistance to the expanded-spectrum oxazolidinone tedizolid. Tedizolid shows improved activity against MRSA and MRCoNS, including cfr-positive isolates (20). To date, the optrA gene has been detected only in a single staphylococcal isolate, namely, a porcine Staphylococcus sciuri isolate (21), where it was located on the 60.5-kb nonconjugative multiresistance plasmid pWo28-3. To see how widespread optrA is among porcine staphylococci, we investigated MRSA and MRCoNS isolates of pig origin from Guangdong Province, China, for the presence and the location of this gene.
MATERIALS AND METHODS
Bacterial isolation and identification.
From June 2013 to June 2014, a total of 910 samples of porcine nasal swabs were collected from three different slaughterhouses (designated A, B, and C). Pigs in these slaughterhouses came from 88 farms in different geographical areas of the Guangdong province of China. The 332 samples in slaughterhouse A originated from 33 farms, the 290 samples in slaughterhouse B from 26 farms, and the 288 samples in slaughterhouse C from 29 farms. MRSA and MRCoNS were isolated and identified as described previously (12, 21). Isolates were screened for the optrA gene by use of primers described previously (16). The bacterial species of the optrA-positive isolates were identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik GmbH, Bremen, Germany), and these results were further confirmed by 16S rRNA sequencing.
Antimicrobial susceptibility testing and screening for resistance genes/mutations.
All optrA-positive isolates were tested for susceptibility to 14 antimicrobial agents by broth microdilution according to CLSI recommendations issued in 2013 (22) and 2016 (23). S. aureus ATCC 29213 served as a quality control strain. Screening for the cfr and cfr(B) genes and for mutations accounting for oxazolidinone resistance was performed using PCR assays described previously (8, 13, 14, 24–27).
Molecular analysis and determination of the location of optrA.
The optrA-carrying isolates were subjected to SmaI pulsed-field gel electrophoresis (PFGE) and dru typing (http://dru-typing.org) (27, 28). PFGE results were analyzed using BioNumerics (version 5.1; Applied Maths, USA). The definition of a PFGE cluster was based on a similarity cutoff of 80% (29). Different PFGE clusters were listed in alphabetical order. S1 nuclease PFGE (S1-PFGE) and Southern blotting were performed to determine the locations of the optrA and cfr genes and the sizes of the optrA-carrying plasmids (21).
Whole-genome sequencing and analysis.
The genetic environments of the optrA gene in selected Staphylococcus isolates were determined by whole-genome sequencing using the Illumina HiSeq 2500 system, which produced 125-bp paired-end reads (Berry Genomics Company, Beijing, China). A draft assembly of the sequences was conducted using CLC Genomics Workbench, version 5 (CLC bio, Aarhus, Denmark); the assembly algorithm works by using de Bruijn graphs (30). All contigs with an average coverage of >100-fold were searched for the optrA gene using BLAST analysis. The regions flanking the optrA gene-carrying contig were identified by a primer walking strategy (16). Sequence analysis was conducted using the ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/) and the BLAST functions (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Accession number(s).
The optrA-carrying sequences in MRCoNS isolates S25-1, MS58-20, S13-1, S031-25, S032-3, MS11-3, and S49-1 were assigned GenBank accession numbers KX447566, KX447567, KX447568, KX447569, KX447570, KX447571, and KX447572, respectively.
RESULTS AND DISCUSSION
Identification of optrA-positive isolates.
A total of 532 methicillin-resistant staphylococci, including 57 MRSA and 475 MRCoNS isolates, were included in this study. Among these, 33 (6.9%) MRCoNS isolates were positive for the optrA gene, and all MRSA isolates were negative. The carriage rates for optrA in staphylococci from slaughterhouses A and B were 9.7% (23/237) and 4.2% (10/238), respectively, while none of the isolates from slaughterhouse C was optrA positive. Further analysis of the origins of the optrA-positive isolates revealed that they were from 12 farms delivering their pigs to slaughterhouse A and 7 farms delivering their pigs to slaughterhouse B. All optrA-harboring MRCoNS were identified as Staphylococcus sciuri.
MICs and detection of resistance genes.
All 33 optrA-positive MRCoNS were resistant to oxacillin, tetracycline, and chloramphenicol and also showed high MICs to tiamulin, virginiamycin M1, and florfenicol. Most of these isolates also exhibited resistance to gentamicin (94%), clindamycin (88%), and erythromycin (88%) and had high spectinomycin MICs (76%), but all of them proved to be susceptible to sulfamethoxazole. The linezolid and tedizolid MICs ranged from 1 to 16 mg/liter and 0.125 to 2 mg/liter, respectively. Among the 33 isolates, only 11 were classified on the basis of their MICs as intermediate or resistant to linezolid (MICs, 4 to 16 mg/liter), while only 4 isolates proved to be nonsusceptible to tedizolid (MICs, ≥1 mg/liter). It is noteworthy that only 3 isolates showed cross-resistance to linezolid and tedizolid, while 5 and 22 isolates were borderline susceptible (MIC, 4 mg/liter) or susceptible (MICs, 1 to 2 mg/liter) to linezolid, respectively. None of the isolates had oxazolidinone resistance-mediating mutations in domain V of the 23S rRNA or in the genes coding for ribosomal proteins L3 and L4.
Comparison of the deduced OptrA amino acid sequences of the 33 isolates with the original OptrA protein from Enterococcus faecalis E349 (designated the wild type) revealed three OptrA variants, all of which differed from the wild type in at least two amino acid positions. Substitutions at positions 176 (Tyr176Asp) and 393 (Gly393Asp) (DD variant) were identified in 27 isolates; alterations at positions 176 (Tyr176Asp), 247 (Asp247Asn), and 393 (Gly393Asp) (DND variant) were found in 4 isolates; and alterations at positions 176 (Tyr176Asp), 247 (Asp247Asn), 393 (Gly393Asp), and 622 (Ile622Met) (DNDM variant) were identified in 2 isolates. Comparative analysis of isolates with the DD variant showed that their linezolid MICs ranged from 1 to 16 mg/liter and their tedizolid MICs ranged from 0.125 to 2 mg/liter. A similar situation was seen for isolates carrying the DND variant, with linezolid MICs ranging from 1 to 16 mg/liter and tedizolid MICs from 0.125 to 0.5 mg/liter. The two isolates with the DNDM variant exhibited linezolid and tedizolid MICs of 1 and 0.125 mg/liter, respectively, which classified these isolates as susceptible to both linezolid and tedizolid. These observations suggested that at least the dominant substitutions at amino acids 176 (Tyr176Asp) and 393 (Gly393Asp), seen in all 33 isolates, had no obvious impact on the linezolid and tedizolid MICs. It should be noted that the three isolates with the highest linezolid MIC of 16 mg/liter also carried the cfr gene.
With regard to resistance genes other than optrA, all 33 isolates carried the mecA gene and the phenicol resistance gene fexA. The novel spectinomycin resistance genes spd and spw were present either alone or in combination in all 33 isolates. Combined resistance to pleuromutilins, lincosamides, and streptogramin A antibiotics was based on the presence of the lsa(E) gene either alone or together with the vga(C) gene in all isolates. Thirty-three isolates were tetracycline resistant and carried the tet(L) (n = 12) or tet(K) (n = 6) gene (Table 1). The gentamicin resistance gene aacA-aphD was detected in 32 isolates. The macrolide-lincosamide-streptogramin B resistance gene erm(C) was present either alone or in combination with erm(B) or with both erm(A) and erm(B) in 29 isolates (Table 1). All 33 isolates were negative for cfr(B), while 5 of them harbored the multiresistance gene cfr. Overlapping PCR assays (31) confirmed that the resistance genes lnu(B) and lsa(E) were present in a gene cluster that often also contained the spectinomycin resistance gene spw. This observation suggests that this cluster not only exists in MRSA (25, 31) but is also widespread in methicillin-resistant S. sciuri. While the resistance genes detected in the MRCoNS isolates can usually explain the observation of resistance phenotypes, there were a few cases in which no gene for a specific resistance property could be detected, such as 15 tetracycline-resistant isolates that were negative for the tet genes detected in this study.
TABLE 1.
Characterization of 33 optrA-carrying MR S. sciuri isolates from pigs in Guangdong
| Isolate | Farm | PFGE type | dru typea | Location of optrAb | OptrA variantc | MIC (mg/liter) of: |
Other resistance phenotyped | Resistance genes | |
|---|---|---|---|---|---|---|---|---|---|
| LZDe | TZDf | ||||||||
| S031-12 | 1 | K | dt11y | P | DND | 16 | 0.5 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, cfr, fexA, aacA-aphD, tet(K), erm(B), erm(C), vga(C), spw, lnu(B), lsa(E) |
| S031-25 | 1 | O | dt11y | C (II) | DD | 8 | 0.5 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(K), erm(A), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S032-2 | 2 | H | dt11y | C (I) | DD | 16 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, cfr, fexA, aacA-aphD, tet(L), erm(B), erm(C), spw, lnu(B), lsa(E) |
| S032-3 | 2 | A | dt10a | C (III) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(K), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S032-5 | 2 | Y2 | dt11y | C (III) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spw, lnu(B), lsa(E) |
| S13-1 | 3 | J1 | dt11y | C (I) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| S13-2 | 3 | G1 | dt11y | P | DD | 2 | 0.125 | OXA CHL FFC AMC GEN TET SPT TIA VIM | mecA, optrA, cfr, fexA, aacA-aphD, spd, spw, lnu(B), vga(C), lsa(E) |
| S22-4 | 4 | P | dt11y | C (I) | DND | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(K), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S24-1 | 5 | C | dt13c | C (IV) | DND | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, spw, lnu(B), vgaC, lsa(E) |
| S25-1 | 6 | X | dt10df* | C (IV) | DD | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S28-1 | 7 | V1 | dt11y | C (IV) | DD | 4 | 0.25 | OXA CHL FFC AMC GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, spd, spw, lnu(B), vga(C), lsa(E) |
| S28-2 | 7 | E | dt11db* | P | DD | 16 | 2 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, cfr, fexA, aacA-aphD, erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| S29-21 | 8 | V3 | dt11y | C (IV) | DD | 2 | 0.5 | OXA CHL FFC AMC GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, spd, spw, lnu(B), lsa(E) |
| S29-5 | 8 | W | dt10a | P | DD | 8 | 2 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, cfr, fexA, aacA-aphD, tet(L), erm(C), spd, spw, lnu(B), lsa(E) |
| S29-6 | 8 | N | dt10a | C (IV) | DD | 2 | 0.5 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S33-14 | 9 | Y2 | dt11y | C (I) | DD | 2 | 0.5 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| S33-15 | 9 | F | dt11y | C (I) | DD | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S33-18 | 9 | L | dt11y | C (I) | DD | 4 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, lnu(B), lsa(E) |
| S33-2 | 9 | J2 | dt11y | C (I) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| S35-13 | 10 | S1 | dt11y | C (IV) | DD | 4 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| S35-16 | 10 | V2 | dt11y | C (I) | DD | 2 | 1 | OXA CHL FFC AMC GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, spd, lnu(B), lsa(E) |
| S39-1 | 11 | I | dt11y | C (IV) | DD | 1 | 0.25 | OXA CHL FFC AMC CLI ERY TET SPT TIA VIM | mecA, optrA, fexA, erm(C), spd, spw, lnu(B), lsa(E) |
| S49-1 | 12 | S2 | dt11y | C (VI) | DND | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spw, lnu(B), vga(C), lsa(E) |
| MS11-3 | 13 | T | dt10at | C (IV) | DD | 8 | 0.5 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(K), erm(B), erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| MS22-9 | 14 | V4 | dt11y | C (I) | DD | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spd, spw, lnu(B), lsa(E) |
| MS25-5 | 15 | M | dt11y | C (I) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spw, lnu(B), vga(C), lsa(E) |
| MS35-4 | 16 | G2 | dt11y | C (I) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, lnu(B), lsa(E) |
| MS35-12 | 16 | R | dt11y | C (I) | DD | 2 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, spw, lnu(B), vga(C), lsa(E) |
| MS37-7 | 17 | U | dt10t | C (IV) | DD | 1 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(L), erm(B), erm(C), spw, lnu(B), vga(C), lsa(E) |
| MS38-4 | 18 | B | dt11y | C (IV) | DD | 4 | 1 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(A), erm(B), erm(C), spd, lnu(B), lsa(E) |
| MS58-2 | 19 | Q | dt11y | C (V) | DNDM | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, lnu(B), lsa(E) |
| MS58-20 | 19 | P | dt11y | C (V) | DNDM | 1 | 0.125 | OXA CHL FFC AMC CLI ERY GEN TET TIA VIM | mecA, optrA, fexA, aacA-aphD, erm(B), erm(C), spd, lnu(B), lsa(E) |
| MS58-23 | 19 | D | dt11y | C (V) | DD | 4 | 0.25 | OXA CHL FFC AMC CLI ERY GEN TET SPT TIA VIM | mecA, optrA, fexA, aacA-aphD, tet(K), erm(A), erm(B), erm(C), spd, lnu(B), lsa(E) |
New dru types (dt11db, 5a-2d-4a-0-3c-5b-3a-2g-4b-4e; dt10df, 5a-3ac-4a-1b-2d-5b-3a-3b-4e-3e) are indicated by asterisks.
P, plasmid; C, chromosome. A Roman numeral in parentheses indicates the type of the genetic environment of optrA that was detected. See Fig. 1.
DD, Tyr176Asp Gly393Asp; DND, Tyr176Asp Asp247Asn Gly393Asp; DNDM, Tyr176Asp Asp247Asn Gly393Asp Ile622Met.
OXA, oxacillin; CHL, chloramphenicol; FFC, florfenicol; AMC, amoxicillin-clavulanic acid; CLI, clindamycin; ERY, erythromycin; GEN, gentamicin; TET, tetracycline; SPT, spectinomycin; TIA, tiamulin; VIM, virginiamycin M1.
LZD, linezolid.
TZD, tedizolid.
Molecular typing and gene location.
PFGE analysis of the 33 MR S. sciuri isolates revealed 25 patterns (Table 1; see also Fig. S1 in the supplemental material), suggesting a high diversity of optrA-positive isolates. As an effective tool applied in previous typing of methicillin-resistant staphylococci (24, 28), dru typing was also conducted to analyze the 33 MR S. sciuri isolates. Among these, five different dru types were identified, including the most common types, dt11y (n = 25 [75.8%]) and dt10a (n = 5 [15.2%]). Single isolates harbored dt13c or the novel dru type dt11db or dt10df (Table 1). S1-PFGE and Southern blotting revealed that optrA was located in the chromosomal DNA of 29 isolates and on a plasmid in the remaining 4 isolates. The cfr gene was colocated on the optrA-carrying plasmid in four isolates and was found in the chromosomal DNA of one isolate.
Genetic environment of optrA on plasmids.
The four MR S. sciuri isolates with plasmid-borne optrA exhibited different PFGE patterns, suggesting that these are epidemiologically unrelated isolates. However, all of them harbored a plasmid of ca. 35 kb. A 17,612-bp contig including the optrA-cfr-aadD-ble-aacA-aphD-fexA resistance gene cluster on each of the four plasmids was indistinguishable from the corresponding region of the 60.5-kb plasmid pWo28-3 (GenBank accession no. KT601170) (16), suggesting that the plasmids found in the four MR S. sciuri isolates are likely to have developed from plasmid pWo28-3 and seem to be disseminated in MR S. sciuri isolates from the Guangdong area.
Genetic environment of optrA in chromosomal DNA.
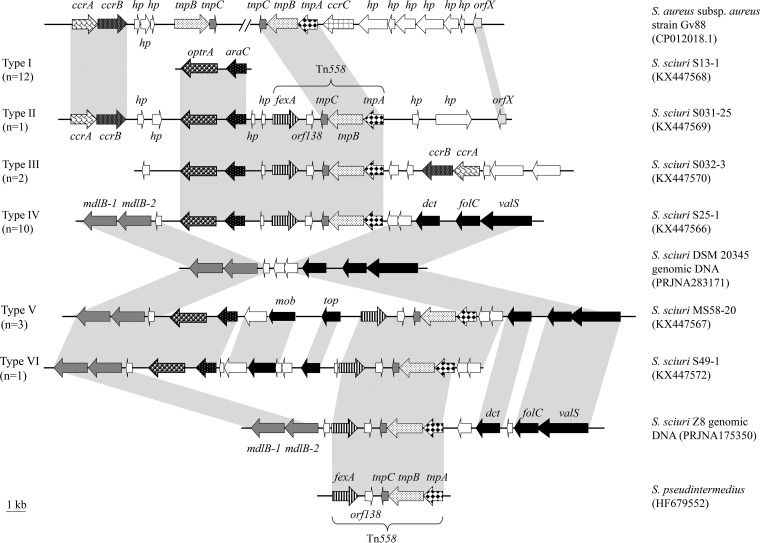
Six types of optrA-carrying fragments (types I to VI), with sizes ranging from 3,455 bp to 28,291 bp, were obtained by whole-genome sequencing of the 29 S. sciuri isolates with chromosomal optrA and were analyzed. Among these, the type I (n = 12) and type IV (n = 10) genetic environments were the most prevalent (Fig. 1). Type I carried the shortest contig, in which only the putative transcriptional regulator gene araC was identified, located immediately upstream of the optrA gene. This araC-optrA core region was also conserved in the other five types. Another commonly identified element was the fexA-carrying transposon Tn558, which was located upstream of araC-optrA in type II to VI genetic environments. Whether or not this transposon plays a role in the dissemination of optrA in S. sciuri remains to be elucidated.
FIG 1.
Schematic presentation of the genetic environments of optrA-flanking regions in the chromosomal DNAs of the 29 MR S. sciuri isolates investigated in this study in comparison to the corresponding chromosomal regions of other S. aureus or S. sciuri isolates. Shaded areas represent regions of >99% nucleotide sequence identity. Arrows indicate the positions and orientations of the genes. Open arrows represent genes coding for hypothetical proteins.
An interesting observation is that a ccr complex with the ccrA-ccrB genes, the products of which exhibited 96.9% and 98.3% amino acid identity to the corresponding proteins of MR S. aureus Gv88 (GenBank accession no. CP012018), was located downstream of optrA-araC in type II genetic environments and upstream of optrA-araC in type III genetic environments. An orfX gene, which is considered the integration site of staphylococcal cassette chromosome mec elements (SCCmec) in the chromosomal DNA of staphylococci, was detected, and its product exhibited 96.6% amino acid identity to that of S. aureus Gv88 (32). Comparative analysis of the optrA-carrying fragments of the 10 isolates harboring type IV genetic environments revealed that an araC-optrA-Tn558 segment was apparently inserted into the corresponding chromosomal region of S. sciuri DSM 20345 (BioProject record no. PRJNA283171) (Fig. 1). This region is characterized by the presence of the mdlB-1 and mdlB-2 genes, coding for a multidrug ABC transporter, and an hp gene, coding for a hypothetical protein, in the upstream part. Downstream of the inserted segment, two hp genes, as well as the dct, folC, and valS genes, coding for a C4-dicarboxylate ABC transporter permease, a folylpolyglutamate synthase, and a valine-tRNA ligase, respectively, were identified. In the three isolates representing the type V genetic environment, the same insertion site was used, but the integrated segment was larger than that in type IV. A segment of 8,018 bp comprising three genes, one gene coding for a hypothetical protein and the mob and top genes, coding for a relaxase/mobilization protein and topoisomerase, respectively, was inserted between the optrA-araC genes and transposon Tn558 (Fig. 1). In the type VI genetic environment, a similar situation was observed. Again, the same insertion site was used, but the fragment between optrA-araC and Tn558 was 6,533 bp long and included five genes coding for hypothetical proteins in addition to the mob and top genes. It should be noted that a complete Tn558 was found to be integrated into the same site in the chromosomal DNA of S. sciuri Z8 (BioProject record no. PRJNA175350). As such, the finding of optrA-araC in connection with Tn558 as well as other genes might be due to several independent integration events.
In conclusion, this is the first large-scale study on the surveillance of the optrA gene in staphylococci of animal origin. Given that all optrA carriers were S. sciuri isolates, it is tempting to speculate about a specific role that this species may play in the dissemination of optrA. Further work is needed to better understand why the same OptrA variant is associated with different linezolid and tedizolid MICs and which other factors may have a positive or a negative impact on the expression of the optrA gene. Since S. sciuri isolates are known to occur in samples from various human, animal, and environmental sources, members of this species may act as a reservoir for virulence and resistance genes, including optrA (33). Further investigations are needed to better understand the role of S. sciuri in the spread of optrA, and surveillance of the transmission and spread of optrA-positive staphylococci is urgently needed.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by grants from the National Natural Science Foundation of China (grants 31472237 and 31370046), the National Basic Research Program of China (grant 2013CB127200), the German Federal Ministry of Education and Research (BMBF) through the German Aerospace Center (DLR) (grant 01KI1301D [MedVet-Staph 2]), and the Chinese Universities Scientific Fund (grant 2015DY004).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01591-16.
REFERENCES
- 1.Woodford N. 2005. Biological counterstrike: antibiotic resistance mechanisms of Gram-positive cocci. Clin Microbiol Infect 11(Suppl 3):S2–S21. doi: 10.1111/j.1469-0691.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argudín MA, Vanderhaeghen W, Butaye P. 2015. Diversity of antimicrobial resistance and virulence genes in methicillin-resistant non-Staphylococcus aureus staphylococci from veal calves. Res Vet Sci 99:10–16. doi: 10.1016/j.rvsc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Feßler AT, Billerbeck C, Kadlec K, Schwarz S. 2010. Identification and characterization of methicillin-resistant coagulase-negative staphylococci from bovine mastitis. J Antimicrob Chemother 65:1576–1582. doi: 10.1093/jac/dkq172. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava K, Zhang Y. 2014. Characterization of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in retail meat. Food Microbiol 42:56–60. doi: 10.1016/j.fm.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Mkrtchyan HV, Cutler RR. 2015. Antibiotic resistance and mecA characterization of coagulase-negative staphylococci isolated from three hotels in London, UK. Front Microbiol 9:947. doi: 10.3389/fmicb.2015.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. 2013. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 68:4–11. doi: 10.1093/jac/dks354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaya A, Kimura A, Sato Y, Ishiwada N, Watanabe M, Matsui M, Shibayama K, Yamamoto T. 2015. Molecular characterization of linezolid-resistant CoNS isolates in Japan. J Antimicrob Chemother 70:658–663. doi: 10.1093/jac/dku443. [DOI] [PubMed] [Google Scholar]
- 9.Mendes RE, Deshpande LM, Jones RN. 2014. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 17:1–12. doi: 10.1016/j.drup.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 68:1697–1706. doi: 10.1093/jac/dkt092. [DOI] [PubMed] [Google Scholar]
- 11.Feßler AT, Calvo N, Gutiérrez N, Bellido JLM, Fajardo M, Garduño E, Monecke S, Ehricht R, Kadlec K, Schwarz S. 2014. cfr-mediated linezolid resistance in methicillin-resistant Staphylococcus aureus and Staphylococcus haemolyticus associated with clinical infections in humans: two case reports. J Antimicrob Chemother 69:268–285. doi: 10.1093/jac/dkt331. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, He T, Schwarz S, Zhao Q, Shen Z, Wu C, Shen J. 2013. Multidrug resistance gene cfr in the methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int J Med Microbiol 303:84–87. doi: 10.1016/j.ijmm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell D, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 59:6256–6261. doi: 10.1128/AAC.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen LH, Vester B. 2015. A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene. Antimicrob Agents Chemother 59:5841–5843. doi: 10.1128/AAC.01274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. 2006. The cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother 50:2500–2505. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 17.Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, Zhang R, Shen J. 2015. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect 21:1095.e1–1095.e4. doi: 10.1016/j.cmi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 18.He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Feßler AT, Zhang R, Wu C, Shen J, Wang Y. 2016. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 71:1466–1473. doi: 10.1093/jac/dkw016. [DOI] [PubMed] [Google Scholar]
- 19.Brenciani A, Morroni G, Vincenzi C, Manso E, Mingoia M, Giovanetti E, Varaldo PE. 2016. Detection in Italy of two clinical Enterococcus faecium isolates carrying both the oxazolidinone and phenicol resistance gene optrA and a silent multiresistance gene cfr. J Antimicrob Chemother 71:1118–1119. doi: 10.1093/jac/dkv438. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Avial I, Culebras E, Betriu C, Morales G, Pena I, Picazo J. 2012. In vitro activity of tedizolid (TR-700) against linezolid-resistant staphylococci. J Antimicrob Chemother 67:167–169. doi: 10.1093/jac/dkr403. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Wang Y, Schwarz S, Cai J, Fan R, Li J, Feßler AT, Zhang R, Wu C, Shen J. 2016. Co-location of the oxazolidinone resistance genes optrA and cfr on a multi-resistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother 71:1474–1478. doi: 10.1093/jac/dkw040. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—4th ed. CLSI document VET01-A4. CLSI, Wayne, PA. [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 26th informational supplement. CLSI document M100-S26. CLSI, Wayne, PA. [Google Scholar]
- 24.Wendlandt S, Kadlec K, Feßler AT, Monecke S, Ehricht R, Giessen AW, Hengeveld PD, Huijsdens X, Schwarz S, Duijkeren E. 2013. Resistance phenotypes and genotypes of methicillin-resistant Staphylococcus aureus isolates from broiler chickens at slaughter and abattoir workers. J Antimicrob Chemother 68:2458–2463. doi: 10.1093/jac/dkt239. [DOI] [PubMed] [Google Scholar]
- 25.Wendlandt S, Li B, Lozano C, Ma Z, Torres C, Schwarz S. 2013. Identification of the novel spectinomycin resistance gene spw in methicillin-resistant and methicillin-susceptible Staphylococcus aureus of human and animal origin. J Antimicrob Chemother 68:1679–1690. doi: 10.1093/jac/dkt081. [DOI] [PubMed] [Google Scholar]
- 26.Wendlandt S, Feßler AT, Monecke S, Ehricht R, Schwarz S, Kadlec K. 2013. The diversity of antimicrobial resistance genes among staphylococci of animal origin. Int J Med Microbiol 303:338–349. doi: 10.1016/j.ijmm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Wu C, Wang Y, Fan R, Schwarz S, Zhang S. 2015. Identification of multiresistance gene cfr in methicillin-resistant Staphylococcus aureus from pigs: plasmid location and integration into a staphylococcal cassette chromosome mec complex. Antimicrob Agents Chemother 59:3641–3644. doi: 10.1128/AAC.00500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadlec K, Schwarz S, Goering RV, Weese JS. 2015. Direct repeat unit (dru) typing of methicillin-resistant Staphylococcus pseudintermedius from dogs and cats. J Clin Microbiol 53:3760–3765. doi: 10.1128/JCM.01850-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDougal LK, Steward IS, Moller JA, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Wendlandt S, Yao J, Liu Y, Zhang Q, Shi Z, Wei J, Shao D, Schwarz S, Wang S, Ma Z. 2013. Detection and new genetic environment of the pleuromutilin-lincosamide-streptogramin A resistance gene lsa(E) in methicillin-resistant Staphylococcus aureus of swine origin. J Antimicrob Chemother 68:1251–1255. doi: 10.1093/jac/dkt015. [DOI] [PubMed] [Google Scholar]
- 32.Harrison EM, Paterson GK, Holden MT, Ba X, Rolo J, Morgan FJ, Pichon B, Kearns A, Zadoks R, Peacock SJ, Parkhill J, Holmes MA. 2014. A novel hybrid SCCmec-mecC region in Staphylococcus sciuri. J Antimicrob Chemother 69:911–918. doi: 10.1093/jac/dkt452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeghaire S, Argudín MA, Feßler AT, Hauschild T, Schwarz S, Butaye P. 2014. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet Microbiol 171:342–356. doi: 10.1016/j.vetmic.2014.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.