Abstract
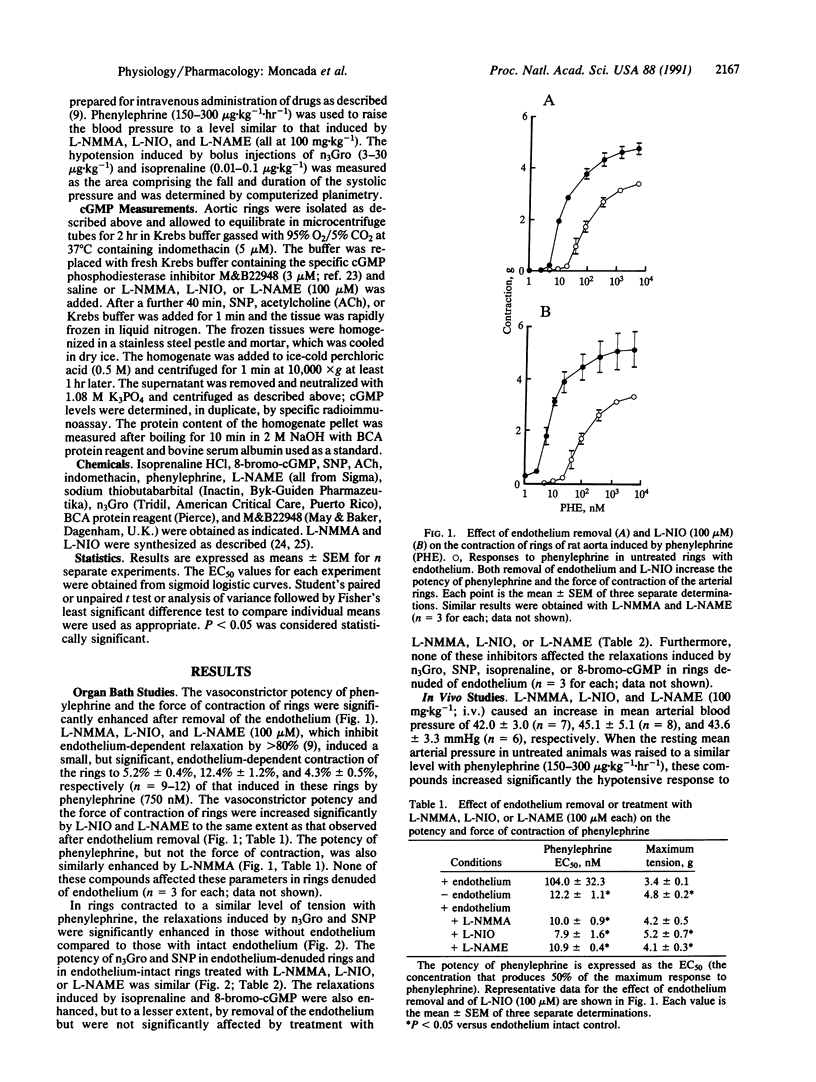
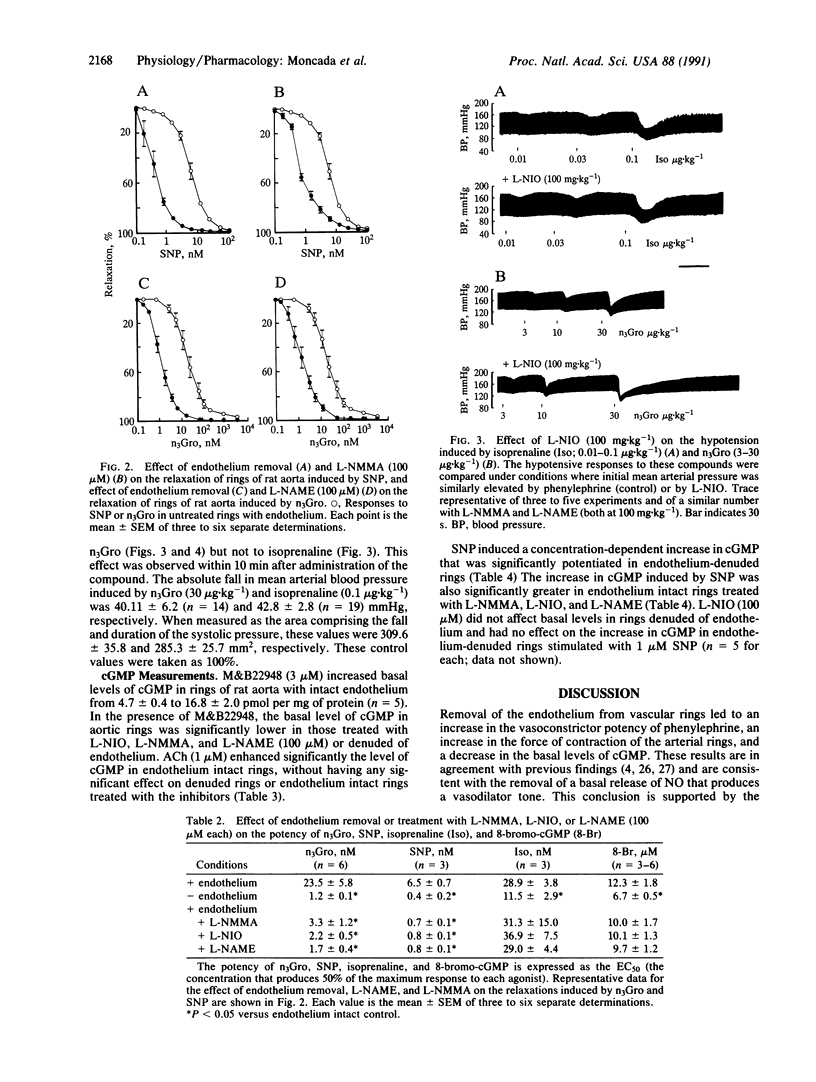
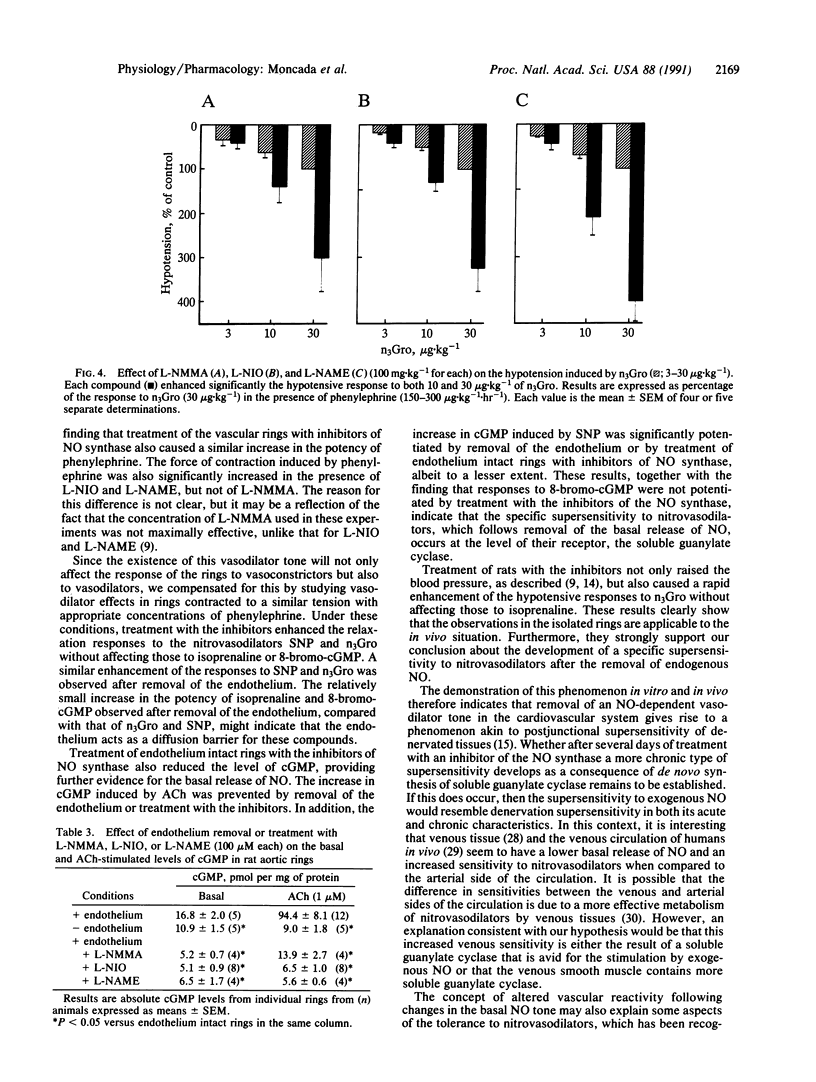
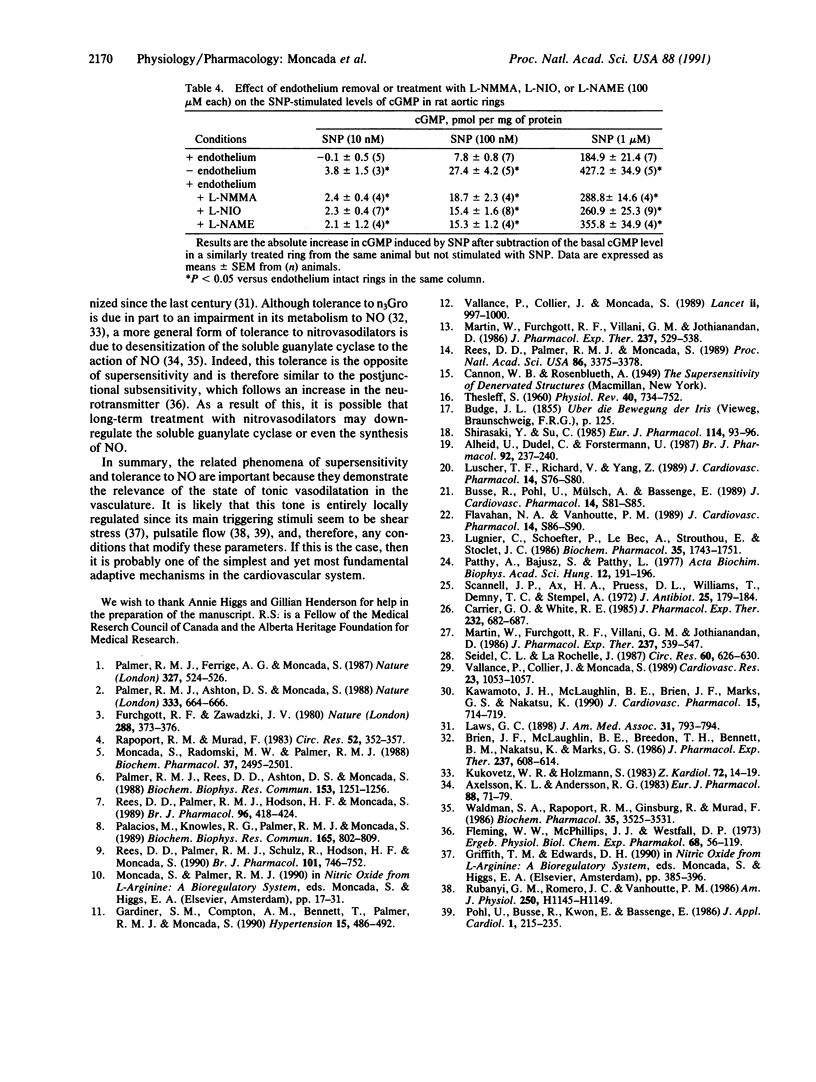
The mechanism of the increased sensitivity to nitrovasodilators after removal of endothelial nitric oxide (NO) was investigated in vitro and in vivo. The vasoconstrictor potency of phenylephrine and the force of contraction of rat isolated aortic rings were significantly enhanced after endothelium removal or treatment with inhibitors of endothelial NO synthase. Furthermore, these procedures led to a significant decrease in the basal levels of cGMP in the vascular rings. Moreover, the potency of glyceryl trinitrate (n3Gro) and sodium nitroprusside (SNP) as relaxing agents and the ability of SNP to induce increases in cGMP in aortic rings were significantly enhanced in those rings denuded of endothelium or treated with the inhibitors. These procedures did not affect the vasodilator actions of isoprenaline or 8-bromo-cGMP. In the anesthetized rat, treatment with the inhibitors enhanced significantly the hypotensive responses to n3Gro without affecting those to isoprenaline. These data indicate that the removal of the basal NO-mediated vasodilator tone in the cardiovascular system leads, at the level of the soluble guanylate cyclase, to a specific supersensitivity to nitrovasodilators in vivo. The existence of such a phenomenon has important implications for understanding the local physiological control of blood flow, its pathological disturbances, and the mechanism of action of nitrovasodilators.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid U., Dudel C., Förstermann U. Selective inhibition by gossypol of endothelium-dependent relaxations augments relaxations to glyceryl trinitrate in rabbit coeliac artery. Br J Pharmacol. 1987 Sep;92(1):237–240. doi: 10.1111/j.1476-5381.1987.tb11317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur J Pharmacol. 1983 Mar 18;88(1):71–79. doi: 10.1016/0014-2999(83)90393-x. [DOI] [PubMed] [Google Scholar]
- Brien J. F., McLaughlin B. E., Breedon T. H., Bennett B. M., Nakatsu K., Marks G. S. Biotransformation of glyceryl trinitrate occurs concurrently with relaxation of rabbit aorta. J Pharmacol Exp Ther. 1986 May;237(2):608–614. [PubMed] [Google Scholar]
- Busse R., Pohl U., Mülsch A., Bassenge E. Modulation of the vasodilator action of SIN-1 by the endothelium. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S81–S85. doi: 10.1097/00005344-198906152-00015. [DOI] [PubMed] [Google Scholar]
- Carrier G. O., White R. E. Enhancement of alpha-1 and alpha-2 adrenergic agonist-induced vasoconstriction by removal of endothelium in rat aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):682–687. [PubMed] [Google Scholar]
- Flavahan N. A., Vanhoutte P. M. Mechanism underlying the inhibitory interaction between the nitrovasodilator SIN-1 and the endothelium. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S86–S90. [PubMed] [Google Scholar]
- Fleming W. W., McPhillips J. J., Westfall D. P. Postjunctional supersensitivity and subsensitivity of excitable tissues to drugs. Ergeb Physiol. 1973;68:55–119. doi: 10.1007/3-540-06238-6_5. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Kawamoto J. H., McLaughlin B. E., Brien J. F., Marks G. S., Nakatsu K. Biotransformation of glyceryl trinitrate and elevation of cyclic GMP precede glyceryl trinitrate-induced vasodilation. J Cardiovasc Pharmacol. 1990 May;15(5):714–719. doi: 10.1097/00005344-199005000-00005. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S. Mechanism of nitrate-induced vasodilation and tolerance. Z Kardiol. 1983;72 (Suppl 3):14–19. [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Richard V., Yang Z. H. Interaction between endothelium-derived nitric oxide and SIN-1 in human and porcine blood vessels. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S76–S80. [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Scannell J. P., Ax H. A., Pruess D. L., Williams T., Demny T. C. Antimetabolites produced by microorganisms. VI. L-N 5 -(1-iminoethyl) ornithine. J Antibiot (Tokyo) 1972 Mar;25(3):179–184. doi: 10.7164/antibiotics.25.179. [DOI] [PubMed] [Google Scholar]
- Seidel C. L., LaRochelle J. Venous and arterial endothelia: different dilator abilities in dog vessels. Circ Res. 1987 Apr;60(4):626–630. doi: 10.1161/01.res.60.4.626. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y., Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985 Aug 7;114(1):93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Effects of motor innervation on the chemical sensitivity of skeletal muscle. Physiol Rev. 1960 Oct;40:734–752. doi: 10.1152/physrev.1960.40.4.734. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989 Dec;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Ginsburg R., Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol. 1986 Oct 15;35(20):3525–3531. doi: 10.1016/0006-2952(86)90622-2. [DOI] [PubMed] [Google Scholar]


