INTRODUCTION
Severe sepsis, characterized by systemic inflammation and acute organ dysfunction due to infection, is estimated to affect 1–3 million patients in the United States per year and to be responsible for 250,000–350,000 in-hospital deaths [1]. An individual’s outcome is difficult to predict in part because it depends on both the patient’s health prior to infection and degree of organ dysfunction after infection. Moreover, while a patient’s health is related to his or her comorbid conditions, it is clear that not all patients with a common comorbid condition are in the same state of health prior to sepsis.
We have previously shown that among patients with hematological malignancies, an inpatient infection in the 90 days prior to a blood-stream infection was common and was associated with greater 30-day mortality after blood-stream infection [2]. We hypothesized that patients who recently survived an infection prior to severe sepsis were in a poorer state of health than those with no history of infection. Thus, we sought to improve the predictive accuracy of the Sequential Organ Failure Assessment (SOFA) score for patients with hematological malignancies and severe sepsis by modifying it to include points for having survived a recent infection.
MATERIALS AND METHODS
Study Location and Patient Population
This study was conducted at the University of Chicago Medical Center in Chicago Illinois, a 568-bed, university-affiliated, urban teaching hospital. Administrative billing records were used to identify all patients with hematological malignancies (ICD-9 codes 200–208) who were admitted to the adult medical ICU between September 1, 2009 and September 1, 2014. Medical records were manually reviewed and patients were included if they had a diagnosis of severe sepsis or septic shock on ICU admission based on consensus definition [3]. Patients were excluded if their malignancy was in complete remission and they were no longer under the care of an oncologist. Only the first ICU admission for severe sepsis for each patient was included as an index case. The University of Chicago Institutional Review Board approved this study and waived the need to obtain informed consent. This study was performed in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its amendments.
Study Design and Data Collection
This study used a retrospective cohort design. Baseline demographic, malignancy, and ICU variables were collected by review of the patients’ electronic medical records, which included nursing documentation, laboratory values, microbiology data, physician notes, radiology reports, and hospital discharge summaries. A patient was determined to have poor performance status if he or she could not perform activities of daily living on hospital admission. The Sequential Organ Failure Assessment (SOFA) score assigns 0–4 points for increasing severity of acute organ failure for each of six organ systems [4]. In this study, we calculated a SOFA score only on the first day of ICU admission. The primary outcome was death during the first 30 days after ICU admission.
For each patient, the most recent inpatient infection that occurred within 90 days prior to ICU admission was recorded. Infections were categorized as bacterial based on the United States Centers for Disease Control and Prevention (CDC) definition [5]. Infections were categorized as fungal if they met “definite” or “probable” criteria based on an International Consensus Definition [6]. Infections were classified as viral if there was clinical suspicion and a positive result on a viral culture, PCR, or histopathology. Inpatient infections treated at outside institutions were also included if documented in the medical records.
Score Derivation
The 50 patients admitted from September 1, 2009 to September 1, 2010 were evaluated to derive the modified SOFA score (SOFA-HM). “Days since most recent infection” was initially treated as a continuous variable; this variable was assigned a value of 91 for patients who did not have an inpatient infection in the 90 days prior to ICU admission. To determine the nature of the relationship between “days since most recent infection” and 30-day mortality independent of SOFA score, we adjusted “days since most recent infection” by dividing it by the expected mortality based on the SOFA score for each patient. We determined that a linear model approximated the relationship between risk of death and days since most recent infection because the addition of “days since most recent infection” squared term did not significantly alter the model (Supplemental Figure 1).
Because there is inherent error in determining the exact day that an infection started, we chose to create three point categories instead of five: 0 points for no infection within 90 days, 2 points for an infection 31–90 days prior to ICU admission, and 4 points for an infection ≤30 days prior to ICU admission. Under univariate analysis, the odds ratios for death for each two-point increase on the “infection” score fell within the 95% confidence intervals of the odds ratios for death for most of the other components of the SOFA score (Supplemental Table 1). Thus, the infection score was given equal weight to the other components of the SOFA score (Table 1). The distributions of prior infection points for patients in the derivation and validation cohorts are displayed in Supplemental Table 2.
Table 1.
The SOFA-HM score combines points for severity of acute organ failure on ICU admission plus additional points for time since most recent infection
| Category | Marker of Dysfunction | Score | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Infection | Days from infection to ICU admission | None or >90 | 31–90 | ≤30 | ||
| Respiratory | PaO2/FiO2, mmHg | >400 | ≤400 | ≤300 | ≤200 | ≤100 |
| Coagulation | Platelets × 1000/μL | > 150 | ≤150 | ≤100 | ≤50 | ≤20 |
| Liver | Bilirubin, mg/dl | <1.2 | 1.2–1.9 | 2.0–5.9 | 6.0–11.9 | >12 |
| Cardiovascular | Hypotension | No Hypotension | Mean Arterial Pressure <70 mmHg | Dopamine ≤5 or Dobutamine (any dose) | Dopamine >5, Epinephrine ≤0.1 or Norepinephrine ≤0.1 | Dopamine >15, Epinephrine >0.1, or Norepinephrine >0.1 |
| Central Nervous System | GCS Scale | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Renal | Creatinine, mg/dl or urine output, ml/day | <1.2 | 1.2–1.9 | 2.0–3.4 | 3.4–4.9 or <500 | >5 or <200 |
Score Validation
For the subsequent 196 patients in the cohort admitted from September 1, 2010 to September 1, 2014, the predictive performance (calibration and discrimination) of the SOFA-HM score was compared to that of the SOFA score and APACHE II score [4, 7]. Calibration is the degree to which actual outcomes match their predicted incidence. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test C-statistic, which compares observed and expected numbers of survivors and deaths across all of the strata of probabilities of death. A high p value (p > 0.05) indicates a good fit for the model. Calibration curves were also created by plotting predicted mortality rates stratified by 20% intervals of mortality risk (x-axis) against observed mortality rates (y-axis). Discrimination is the degree to which a score is able to identify patients at highest risk for death. The discrimination was determined by calculating the area under the receiver operating characteristic curve (AUROC) and its 95% confidence interval (CI).
Data Analysis
Continuous variables were reported as medians with 25th and 75th percentiles. The Student’s t-test, Mann–Whitney U test, Chi-square test, or Fisher exact test were used in bivariate testing, as appropriate, to determine if there were significant differences between groups. Logistic regression analysis was used to identify risk factors present on ICU admission that were independently associated with 30-day mortality after adjusting for age and SOFA-HM score. Initial models included all recorded variables; backward selection was then employed to determine a final model. All tests were two-sided and a p-value ≤0.05 was considered to indicate statistical significance. All analyses were performed with STATA 13.1 (StataCorp, College Station, TX).
RESULTS
Between September 1, 2009 and September 1, 2014, there were 433 distinct patients with hematological malignancies admitted to the medical ICU. There were 27 (6.2%) patients excluded because their malignancies were in complete remission and were no longer being managed. There were 246 (57%) who met criteria for severe sepsis.
Of the patients with severe sepsis, there were 98 patients (40%) who had an inpatient infection in the 90 days prior to ICU admission for severe sepsis. Of these prior infections, 11 (11%) occurred at outside hospitals. The most common organ systems involved were respiratory and primary bloodstream for both index cases and prior infections (Table 2). When examining the derivation and validation cohorts as a group, each “two point” increase in infection score was an independent risk factor for death. The magnitude of the effects was similar to that of the respiratory, coagulation, and central nervous system components of the SOFA score (Table 3). The liver, cardiovascular, and renal scores were not independently associated with 30-day mortality.
Table 2.
Types of infections by organ system and organ system for 246 patients (50 from derivation cohort and 196 from validation cohort)
| Index Severe Sepsis Case N=246 |
Types of Infections that occurred prior to ICU Admission* | ||
|---|---|---|---|
| Organ | Respiratory n (%) | 116 (47) | 36 (15) |
| Blood-stream n (%) | 65 (26) | 30 (12) | |
| Gastrointestinal n (%) | 17 (6.9) | 17 (6.9) | |
| Skin/Soft Tissue n (%) | 7 (2.9) | 9 (3.7) | |
| Genitourinary n (%) | 5 (2.0) | 4 (1.6) | |
| Other n (%) | 2 (0.8) | 0 (0) | |
| Undetermined n (%) | 34 (14) | 2 (0.81) | |
| None n (%) | n/a | 148 (60) | |
| Organism | S. aureus n (%) | 14 (5.7) | 4 (1.6) |
| Enterococcus sp n (%) | 9 (3.7) | 6 (2.4) | |
| Other gram positive bacterial n (%) | 24 (9.8) | 9 (3.7) | |
| Pseudomonas sp n (%) | 19 (7.7) | 3 (1.2) | |
| Klebsiella sp. n (%) | 7 (2.9) | 7 (2.8) | |
| E. coli n (%) | 7 (2.9) | 5(2.03 | |
| Other gram negative bacteria n (%) | 8 (3.3) | 4 (1.6) | |
| Polymicrobial bacterial n (%) | 11 (4.5) | 3 (1.2) | |
| Clostridium difficile n (%) | 11 (4.5) | 14 (5.7) | |
| Viral n (%) | 16 (6.5) | 10 (4.1) | |
| Fungal n (%) | 13 (5.3) | 13 (5.3) | |
| Mycobacterial n (%) | 1 (0.4) | 1 (0.41) | |
| Undetermined n (%) | 106 (43) | 19 (7.7) | |
| None n (%) | n/a | 148 (60) | |
98 patients had infections in the 90 days prior to admission for severe sepsis
Table 3.
Multivariable model with 30-day mortality as the outcome and the individual components of the SOFA-HM score as predictors for all 246 patients (derivation + validation cohorts). For the components of the SOFA score, the patients with “1–2” points were grouped and patients with “3–4” points were grouped.
| Score | Points | OR | 95% CI | p value |
|---|---|---|---|---|
| Infection | 0 | – | – | – |
| 2 | 1.50 | 0.68–3.35 | 0.32 | |
| 4 | 5.28 | 2.21–12.6 | <0.001 | |
| Respiratory | 0 | – | – | – |
| 1–2 | 2.46 | 1.17–5.14 | 0.02 | |
| 3–4 | 4.30 | 1.77–10.4 | 0.001 | |
| Coagulation | 0 | – | – | – |
| 1–2 | 2.07 | 0.76–5.65 | 0.16 | |
| 3–4 | 4.69 | 1.81–12.1 | 0.001 | |
| Liver | 0 | – | – | – |
| 1–2 | 0.94 | 0.40–2.24 | 0.89 | |
| 3–4 | 0.63 | 0.22–1.81 | 0.39 | |
| Cardiovascular | 0 | – | – | – |
| 1–2 | 0.90 | 0.41–1.96 | 0.78 | |
| 3–4 | 0.53 | 0.25–1.12 | 0.1 | |
| Central Nervous System | 0 | – | – | – |
| 1–2 | 3.78 | 1.96–7.3 | <0.001 | |
| 3–4 | 22.2 | 4.05–121 | <0.001 | |
| Renal | 0 | – | – | – |
| 1–2 | 1.27 | 0.63–2.57 | 0.51 | |
| 3–4 | 1.76 | 0.71–4.41 | 0.22 |
Figure 1 displays the calibration curves for the SOFA-HM, SOFA, and APACHE II scores. Hosmer-Lemeshow C-statistic p values for the SOFA-HM, SOFA, and APACHE II scores were 0.42, 0.25, and 0.31 respectively, indicating that all scores fit the data well. There was a more even distribution of numbers of patients across all strata of expected mortality for the SOFA-HM score compared to the SOFA and APACHE II scores. The SOFA-HM score discriminated survivors from non-survivors significantly better than SOFA score and APACHE II. The SOFA-HM score had an AUROC of 0.73 (95% CI 0.66–0.80). The SOFA score had an AUROC of 0.68 (95% CI 0.61–0.76, p=0.005 for difference with SOFA-HM) and the APACHE II score had an AUROC of 0.65 (95% CI 0.58–0.73, p=0.04 for difference with SOFA-HM) (Figure 2).
Figure 1.
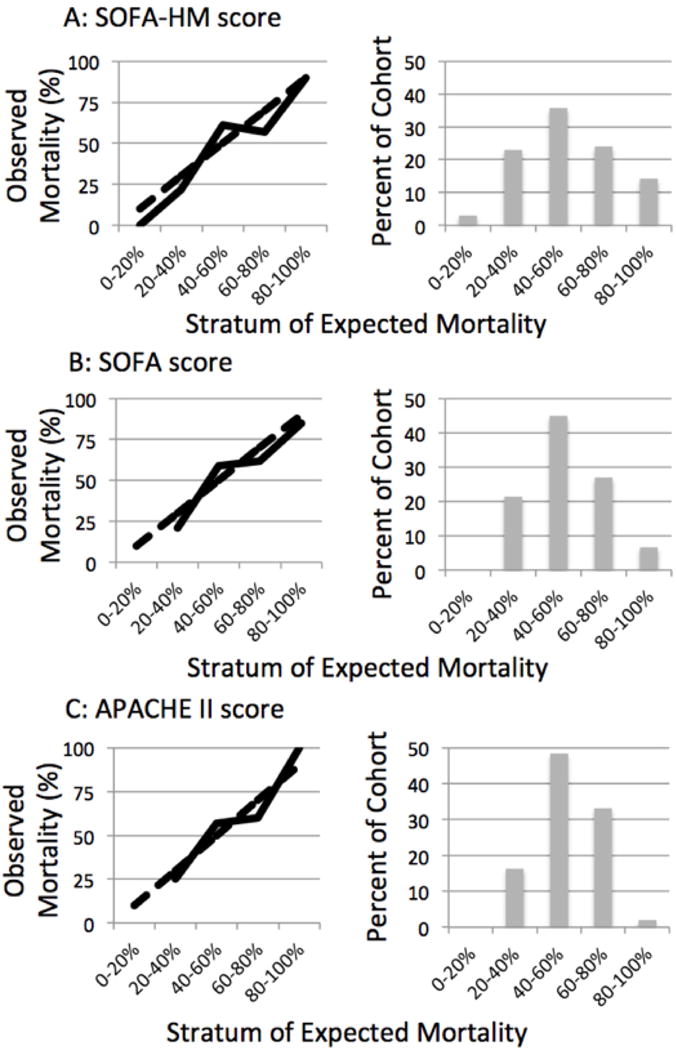
Calibration curves for the SOFA-HM, SOFA, and APACHE II scores for the 196 patients in the validation cohort. (Solid line – mean observed mortality. Dashed line – expected mortality)
Figure 2.
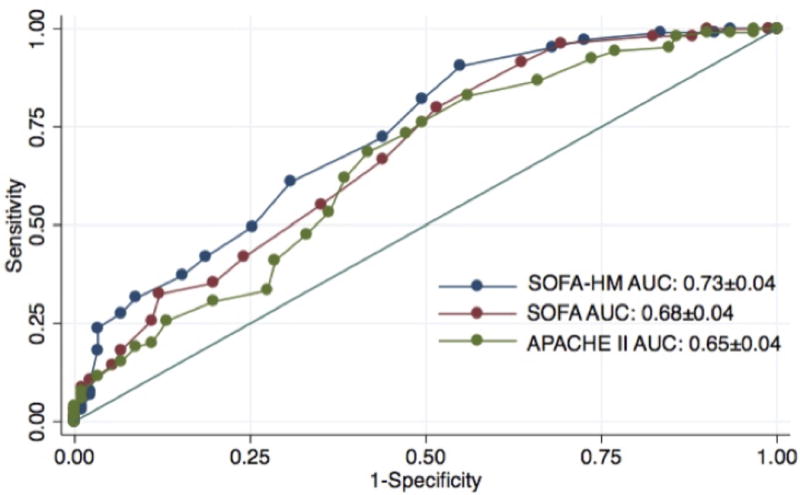
Receiver Operating Characteristic (ROC) curves for the SOFA-HM, SOFA, and APACHE II scores for the 196 patients in the validation cohort.
In the validation cohort, there were 105 patients (54%) who died during the first 30 days after ICU admission (Table 4). Under bivariate analysis, a relapsed/progressive hematological malignancy and a greater number of days in the hospital prior to ICU admission were positively and significantly associated with mortality (p<0.05). Patients who died were more likely to require invasive mechanical ventilation and vasoactive agents (p<0.05). After adjusting for SOFA-HM score and age, only increased days from hospital to ICU admission was significantly associated with 30-day mortality (Table 5).
Table 4.
Bivariate associations between patient characteristics and mortality for the 196 patients in the validation cohort.
| Died during first 30 days N=105 |
Alive N=105 |
p value | ||
|---|---|---|---|---|
| Demographic | Age median [IQR] | 62 [50, 70] | 59 [50, 67] | 0.12 |
| Male n(%) | 61 (58) | 54 (59) | 0.86 | |
| White n(%) | 62 (59) | 57 (63) | 0.34 | |
| Black n(%) | 31 (30) | 29 (32) | ||
| Other race n(%) | 12 (11) | 5 (5.5) | ||
| Hematological Malignancy Variables | Acute Myeloid Leukemia n(%) | 48 (46) | 34 (37) | 0.76 |
| Acute Lymphocytic Leukemia n(%) | 13 (12) | 9 (9.9) | ||
| Non-Hodgkin Lymphoma n(%) | 19 (18) | 22 (24) | ||
| Hodgkin Lymphoma n(%) | 2 (1.9) | 5 (5.5) | ||
| Multiple Myeloma n(%) | 11 (10.5) | 9 (9.9) | ||
| Myelodysplastic Syndrome/Myelofibrosis n(%) | 1 (1.0) | 2 (2.2) | ||
| Chronic Myeloid Leukemia n(%) | 4 (3.8) | 4 (4.4) | ||
| Chronic Lymphocytic Leukemia n(%) | 7 (6.7) | 6 (6.6) | ||
| Months Since Diagnosis | 13 [3.2, 40] | 8.7 [2.0, 24] | 0.19 | |
| Allogenieic SCT n(%) | 40 (38) | 33 (36) | 0.79 | |
| SCT complication n(%) | 17 (58) | 16 (52) | 0.61 | |
| Days since SCT median [IQR] | 77 [31, 217] | 88 [18, 201] | 0.91 | |
| Chemotherapy in previous 2 months n(%) | 79 (75) | 64 (71) | 0.52 | |
| Neutrophils < 500 cells/μL on ICU admission n(%) | 43 (42) | 39 (43) | 0.88 | |
| Poor performance status n(%) | 16 (15) | 11 (12) | 0.52 | |
| Relapsed/Progressive Disease n(%) | 65 (62) | 39 (43) | 0.008 | |
| Other Chronic Diseases | Lung disease n(%) | 14 (13) | 15 (17) | 0.51 |
| Cardiovascular disease n(%) | 26 (25) | 24 (26) | 0.8 | |
| Diabetes n(%) | 25 (24) | 23 (25) | 0.81 | |
| Additional malignancy n(%) | 28 (27) | 20 (22) | 0.45 | |
| Chronic Kidney Disease n(%) | 10 (9.5) | 9 (9.9) | 0.93 | |
| Deep Venous Thrombosis/Pulmonary n(%)Embolus | 10 (9.5) | 12 (13) | 0.42 | |
| Autoimmune n(%) | 7 (6.7) | 5 (5.5) | 0.78 | |
| Liver disease n(%) | 1 (0.95) | 5 (5.5) | 0.1 | |
| Sleep disordered breathing n(%) | 5 (4.8) | 7 (7.7) | 0.55 | |
| ICU Variables | Days from hospital admission to ICU admission median [IQR] | 4 [0, 14] | 1 [0, 10] | 0.02 |
| ICU length of stay median [IQR] | 5 [1, 10] | 4 [2, 9] | 0.77 | |
| Vasoactive agents n(%) | 65 (62) | 39 (43) | 0.01 | |
| Acute renal replacement therapy n(%) | 20 (19) | 9 (9.9) | 0.11 | |
| Invasive mechanical ventilation n(%) | 71 (68) | 26 (29) | <0.001 | |
| Ventilator Days median [IQR] | 1 [0, 5] | 0 [0, 2] | <0.001 | |
| SOFA Score median [IQR] | 9 [7, 13] | 7 [4, 9] | <0.001 | |
| SOFA-HM Score median [IQR] | 10 [8, 15] | 7 [4, 11] | <0.001 | |
| APACHE II Score median [IQR] | 26 [22, 31] | 21 [18, 29] | <0.001 | |
Table 5.
Multivariable model for the 196 patients in the validation cohort.
| OR | 95% CI | p value | |
|---|---|---|---|
| SOFA-HM | 1.21 | 1.12–1.30 | <0.001 |
| Age | 1.03 | 1.00–1.05 | 0.02 |
| Days from Hospital to ICU admission* | 1.03 | 1.00–1.06 | 0.03 |
The interaction term between SOFA-HM and days from hospital to ICU admission was non-significant and not included in the model (p=0.58).
DISCUSSION
In this study, we used a novel approach to improving the predictive accuracy of an ICU scoring system for patients with hematological malignancies. Previous investigators have modified scoring systems by altering the weights of components or by combining aspects of different scoring systems [8, 9]. However, we hypothesized that a main limitation of these scoring systems is that they are missing variables for populations at high risk for death from sepsis.
As a whole, patients with hematological malignancies are among the highest risk for developing and dying from severe infections [10]. Our mortality rate of 54% is similar to that described in previous publications [11–14]. However, all patients with hematological malignancies do not have the same baseline risk of death from severe sepsis. In our study, ICU mortality was inversely associated with time since most recent infection, independent of the degree of acute organ failure. As a result, we were able to derive a point system for the prior infection variable and incorporate it into the SOFA score. It is likely that events that occur in the hospital prior to an ICU admission are prognostic for high-risk populations and would add to the predictive accuracy of ICU scoring systems. These events could include deep vein thrombosis, arrhythmias/cardiac events, and pressure ulcers.
There are two ways that the SOFA-HM performed better than the SOFA and APACHE II scores. First, the SOFA-HM score discriminated survivors from non-survivors significantly better than the SOFA and APACHE II. Second, the SOFA-HM had the most even distribution of scores across all strata of expected mortality. ICU scoring systems would likely have more clinical use if greater percentages of patients had either very high or very low expected mortalities. The main limitation of the SOFA-HM score is that it could be challenging to automate. It relies on a clinician having access to and reviewing medical records for a patient.
After controlling for age and SOFA-HM score, only time from hospital admission to ICU admission remained significantly associated with mortality. Other studies of critically patients with hematological malignancies have identified this as a poor prognostic factor as well [15, 16]. We also confirm the findings of previous investigators that variables associated with malignancy (type of malignancy, recent chemotherapy administration, neutrophil count) are not associated with mortality from critical illnesses [11, 17, 18]. This surprising finding could be explained by the fact that patients must be in a relatively “good” state of health in order to be eligible for chemotherapy. In addition, patients with neutropenia may have a more transient risk for severe infection than patients who are immunosuppressed for other reasons.
As patients with severe sepsis have high prevalence of chronic diseases, they often require medical care in the months leading up to a hospitalization for severe sepsis. Prescott et al. estimated that in the year prior to an episode of severe sepsis, patients had an average of 1.44 hospitalizations and 24.2 days in the hospital per patient-year [19]. Although the need for frequent healthcare likely signifies a state of poor health, prior healthcare utilization alone does not affect outcomes from sepsis independent of physiological-based scores [20]. Our findings suggest that in order to discover significant associations between prior healthcare use and outcomes from sepsis, patients should be grouped by comorbid conditions and the focus should be on infectious instead of non-infectious reasons for hospitalization.
There are multiple ways that a prior infection could prognosticate a worse outcome from a future episode of severe sepsis. A prior infection could be a marker for an unmeasured poor prognostic factor for patients with hematological malignancies. For instance, a history of recurrent infections may delay chemotherapy or alter goals of care for patients with aggressive malignancies. It is also possible that patients with prior infections are at higher risk for subsequent infections by virulent, antibiotic-resistant organism than those with no prior infection history. Finally, patients who survive an infection may enter an “immune recovery” phase; patients who develop severe sepsis during this recovery phase may have the highest risk for death [21].
Clearly, the main limitation of this study was that the sample size was small. Although we derived the SOFA-HM score among a subgroup of 50 patients, we demonstrated that the “infection score” influenced mortality to a similar extent as the other components of the SOFA score in the whole 246-patient cohort. Another limitation was that this study was conducted at a single healthcare center, which potentially affects its generalizability. Clinicians in our ICU care for a large volume of critically ill patients with hematological malignancies, many of whom have relapsed or progressive diseases. Thus, we suspect that the SOFA-HM might not be more accurate than the SOFA score in healthcare systems where most patients are newly diagnosed with cancer and have less prior nosocomial exposure.
Overall, our findings support the hypothesis that a patient’s baseline health influences his or her outcomes from severe sepsis independent of severity of acute illness. A major weakness of ICU scoring systems is that even though they assign points for comorbid conditions, they do not adequately account for patient's chronic health status. Among patient populations that are high risk for developing severe sepsis, history of recent infection should be investigated as a surrogate for poor health. We suggest that clinicians who work at centers that care for many patients with hematological malignancies investigate the predictive ability of SOFA-HM score at their institutions. If the SOFA-HM predicts outcomes better than other scoring systems, as it did in our study, it should be used to benchmark mortality rates over time and stratify patients who are enrolled in clinical trials.
Supplementary Material
Supplemental Figure 1 – Mortality vs. Days since most recent infection (adjusted for expected mortality) among the 50 patients in the derivation cohort.
Acknowledgments
Research Support:
Research Training in Respiratory Biology (2 T32 HL007605-28)
Dr. Churpek has a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients.
Abbreviations List
- SOFA
Sequential Organ Failure Assessment
- APACHE
Acute Physiology and Chronic Health Evaluation
- AUROC
Area Under the Receiver Operating Characteristic curve
Footnotes
Conflict of Interest
No other authors have conflicts of interest that could lead to bias.
AUTHORS CONTRIBUTIONS
JAG: contributed to the conception and design of the study; data collection and statistical analysis; drafting, critical revision, reading, and approval of the manuscript.
MZD: contributed to the conception and design of the study; critical revision, reading, and approval of the manuscript.
MMC: contributed to the statistical analysis; drafting, critical revision, reading, and approval of the manuscript.
DLP: contributed to the conception and design of the study; critical revision, reading, and approval of the manuscript.
JBH: contributed to the conception and design of the study; critical revision, reading, and approval of the manuscript.
JPK: contributed to the conception and design of the study; critical revision, reading, and approval of the manuscript.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Critical care medicine. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JA, David MZ, Pitrak DL, Hall JB, Kress JP. Prior infections are associated with increased mortality from subsequent blood-stream infections among patients with hematological malignancies. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2014 doi: 10.1007/s10096-014-2114-y. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive care medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 5.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American journal of infection control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 7.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 8.Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care. 2008;12(6):R161. doi: 10.1186/cc7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirracchio R, Petersen ML, Carone M, Rigon MR, Chevret S, van der Laan MJ. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): a population-based study. The Lancet Respiratory medicine. 2014 doi: 10.1016/S2213-2600(14)70239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5):R291–298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit DD, Depuydt PO, Peleman RA, Offner FC, Vandewoude KH, Vogelaers DP, Blot SI, Noens LA, Colardyn FA, Decruyenaere JM. Documented and clinically suspected bacterial infection precipitating intensive care unit admission in patients with hematological malignancies: impact on outcome. Intensive care medicine. 2005;31(7):934–942. doi: 10.1007/s00134-005-2599-z. [DOI] [PubMed] [Google Scholar]
- 12.de Montmollin E, Tandjaoui-Lambiotte Y, Legrand M, Lambert J, Mokart D, Kouatchet A, Lemiale V, Pene F, Bruneel F, Vincent F, et al. Outcomes in critically ill cancer patients with septic shock of pulmonary origin. Shock. 2013;39(3):250–254. doi: 10.1097/SHK.0b013e3182866d32. [DOI] [PubMed] [Google Scholar]
- 13.Larche J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, Darmon M, Le Gall JR, Schlemmer B. Improved survival of critically ill cancer patients with septic shock. Intensive care medicine. 2003;29(10):1688–1695. doi: 10.1007/s00134-003-1957-y. [DOI] [PubMed] [Google Scholar]
- 14.Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, Lemiale V, Seguin A, Darmon M, Schlemmer B, et al. Survival in neutropenic patients with severe sepsis or septic shock. Critical care medicine. 2012;40(1):43–49. doi: 10.1097/CCM.0b013e31822b50c2. [DOI] [PubMed] [Google Scholar]
- 15.Azoulay E, Mokart D, Pene F, Lambert J, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Laisne LM, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium–a groupe de recherche respiratoire en reanimation onco-hematologique study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(22):2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 16.Hampshire PA, Welch CA, McCrossan LA, Francis K, Harrison DA. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2009;13(4):R137. doi: 10.1186/cc8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A, Installe E. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Critical care medicine. 2002;30(10):2260–2270. doi: 10.1097/00003246-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Vandijck DM, Benoit DD, Depuydt PO, Offner FC, Blot SI, Van Tilborgh AK, Nollet J, Steel E, Noens LA, Decruyenaere JM. Impact of recent intravenous chemotherapy on outcome in severe sepsis and septic shock patients with hematological malignancies. Intensive care medicine. 2008;34(5):847–855. doi: 10.1007/s00134-008-1002-2. [DOI] [PubMed] [Google Scholar]
- 19.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. American journal of respiratory and critical care medicine. 2014;190(1):62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Prior healthcare utilization as a predictor of survival for medical intensive care unit patients. Critical care medicine. 2000;28(8):3053–3059. doi: 10.1097/00003246-200008000-00063. [DOI] [PubMed] [Google Scholar]
- 21.Leentjens J, Kox M, van der Hoeven J, Netea MG, Pickkers P. Immunotherapy for the Adjunctive Treatment of Sepsis: From Immunosuppression to Immunostimulation. Time for a Paradigm Change? American journal of respiratory and critical care medicine. 2013 doi: 10.1164/rccm.201301-0036CP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Mortality vs. Days since most recent infection (adjusted for expected mortality) among the 50 patients in the derivation cohort.


