Figure 1. When proteasomes are completely inhibited, Nrf1 can still be processed, but becomes insoluble and leaves solution.
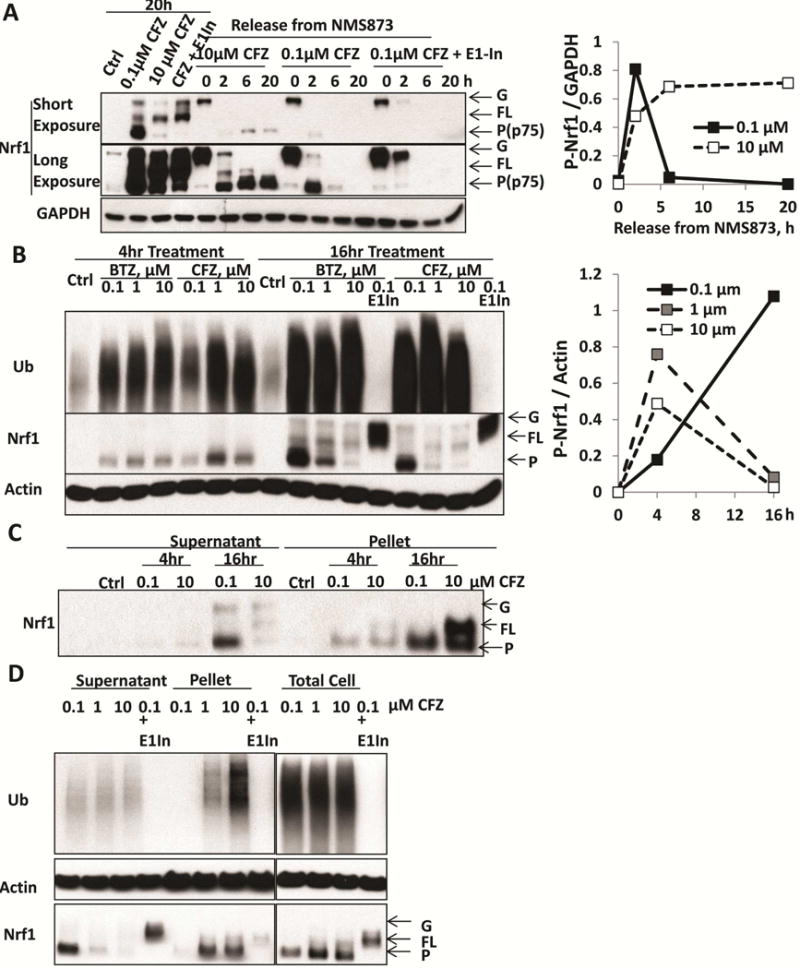
A. HEK293A cells were treated for 20h with 0.1 or 10 μM carfilzomib (CFZ), or with a combination of 0.1 μM CFZ and 0.5 μM E1 inhibitor (ML00603997, E1-In). In addition, to monitor the processing of glycosylated precursor (G-Nrf1), the cells were first treated with the p97 inhibitor NMS873 (10 μM) for 2h to cause endogenous Nrf1 to accumulate as the glycosylated precursor. NMS873 was then removed and the cells cultured for 2, 6, and 20h in the presence of cycloheximide to inhibit new protein synthesis (Supplemental Fig S1B). Following NMS873 removal, cells were treated with 0.1 or 10 μM CFZ, or with a combination of 0.1 μM CFZ and 0.5 μM E1-inhibitor in order to determine whether processing of glycosylated Nrf1 required proteasome activity (completely blocked by 10 μM CFZ) or ubiquitination (by E1-In). The levels of glycosylated (G), deglycosylated full-length (FL), and processed (P) forms (also termed p75 [2]) of Nrf1 were assayed by western blotting (Left). Densitometry was performed to quantify the levels of processed (P-Nrf1, p75) in cells undergoing NMS873-release with co-treatment of 0.1 or 10 μM CFZ and normalized with GAPDH in right panel.
B. HEK293F cells were treated with 0.1, 1, or 10 μM BTZ or CFZ for 4 or 16h. These cells were also treated with 0.1 μM BTZ or CFZ together with 0.5 μM E1-In for 16h. The levels of glycosylated, full-length, and processed forms of Nrf1 in the cell lysate were measured by western blotting (Left). Quantifications of the levels of processed Nrf1 (P-Nrf1, normalized to actin) in CFZ-treated cells are shown in the right panel.
C. HEK293F cells were treated with 0.1 or 10 μM CFZ for 4 or 16h. The 10,000 × g pellet fraction of the cells after lysis with buffer containing 1% TX-100 was collected by centrifugation for 10min and solubilized in 2% SDS. The levels of G, FL, P forms of Nrf1 in this pellet fraction were measured by western blotting.
D. HEK293F cells were treated for 16h with 0.1, 1, or 10 μM CFZ, or a combination of 0.1 μM CFZ and 0.5 μM E1 inhibitor. The levels of G, FL, P forms of Nrf1 in the lysate and pellet fractions, or in the total cell protein (by directly lysis of cells in 2% SDS) were measured by Western blotting.
