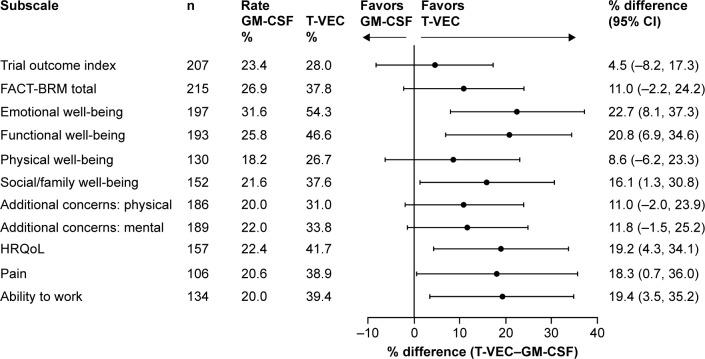
Figure 2.
Improvement in HRQoL assessed by FACT-BRM during treatment with talimogene laherparepvec and GM-CSF in the stage IIIB/C or IVM1a OPTiM subpopulation (ITT).a
Notes: aScores from unscheduled visits were not included. A patient is considered evaluable for a domain if baseline score is not the best score, there is room for evaluable improvement and there is at least one post-baseline score. TOI and total improvements are defined as ≥5-point score increase from baseline with a ≥1 cycle duration. HRQoL, pain and work improvements are defined as ≥1-point score increase from baseline with a ≥1 cycle duration. All other subscales are based on a ≥2-point score increase from baseline with a ≥1 cycle duration.
Abbreviations: CI, confidence interval; FACT-BRM, Functional Assessment of Cancer Therapy-Biologic Response Modifier; GM-CSF, granulocyte-macrophage colony-stimulating factor; HRQoL, health-related quality of life; TOI, trial outcome index; T-VEC, talimogene laherparepvec.