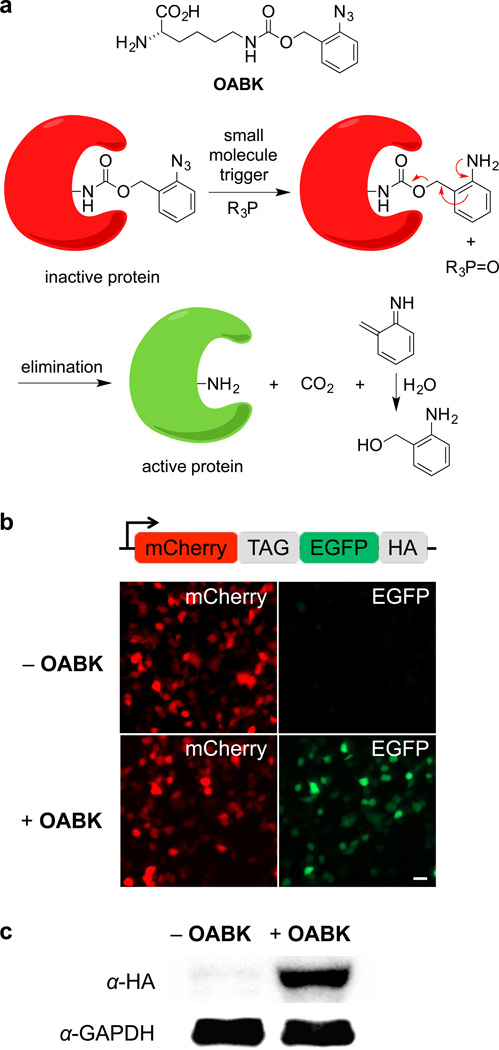
Figure 1. Staudinger reduction-based protein activation through genetic encoding of OABK in living cells.
(a) Structure of OABK and schematic of the phosphine-triggered protein activation through protecting group removal via a Staudinger reduction. OABK is site-specifically incorporated into a protein of interest via genetic code expansion, and then the protein is deprotected and activated through a phosphine-induced Staudinger reduction followed by a 1,4-elimination of the azidobenzyl group. (b) Micrographs confirming amino acid-dependent incorporation of OABK into mCherry-TAG-EGFP-HA in HEK293T cells. EGFP fluorescence was observed only in the presence of OABK, due to suppression of the TAG stop codon. Scale bar represents 20 µm. (c) Confirmation of OABK-dependent full-length protein expression through an anti-HA Western blot.