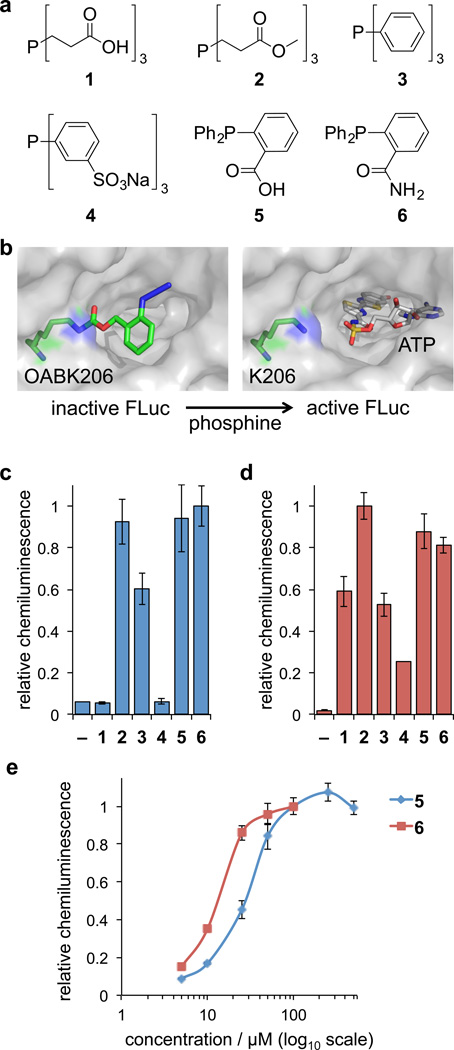
Figure 3. Phosphine screening for Staudinger reduction-mediated deprotection and activation of firefly luciferase in cells and cell lysates.
(a) Structures of the six phosphine derivatives TCEP (1), TCEP ester (2), TPP (3), TPPTS (4), 2DPBA (5), and 2DPBM (6). (b) Model of FLuc containing OABK at position K206, thereby blocking access of ATP to the catalytic site until the azidobenzyloxycarbonyl group is cleaved by the small molecule trigger (based on PDB 2D1S). (c) Initial screening of phosphines 1–6 (at maximum concentrations based on the solubility and cell viability, see Supplementary Figure 3) in order to identify efficient activators of OABK-FLuc in HEK293T cells and in (d) cell lysates. Error bars represent standard deviations from three independent experiments. (e) Live cell dose-dependent luciferase assays were conducted for the most efficient small molecule triggers 5 (5, 10, 25, 50, 100, 250, 500 µM) and 6 (5, 10, 25, 50, 100 µM), revealing 6 as the most active phosphine. The solubility of 6 in an aqueous environment limits its application to ≤100 µM. Error bars represent standard deviations from three independent experiments.