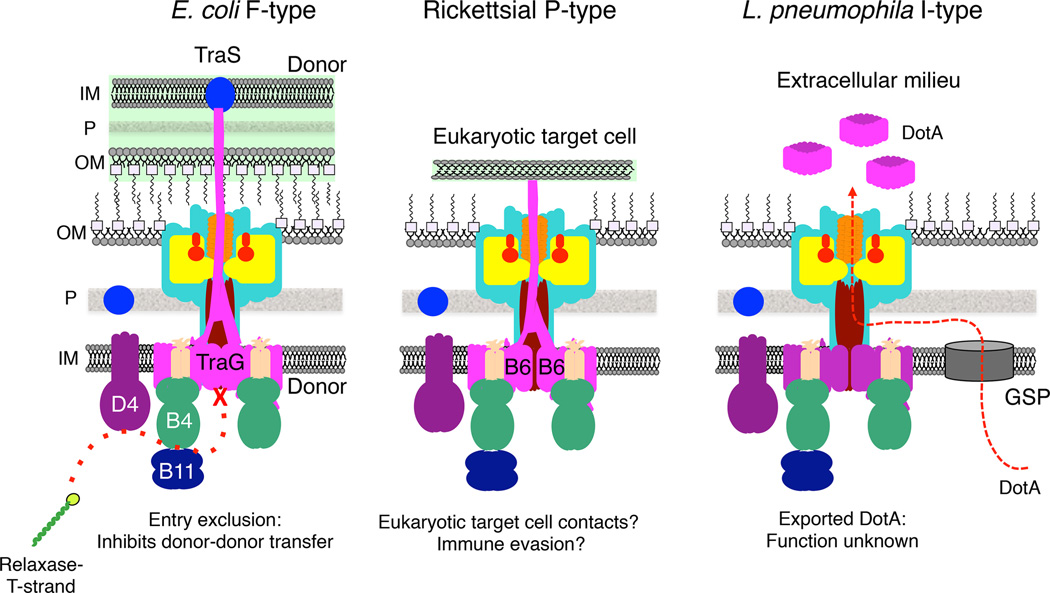
Figure 4. Adaptations of the IMC: VirB6-like subunits.
Polytopic VirB6 subunits with lengths of ~300 residues are components of the IMC. Many T4SSs have larger forms of these subunits, designated extended-VirB6. These forms of VirB6 are composed of a polytopic VirB6-like domain and a hydrophilic domain as large as ~1,000 residues. (Left) F-type F plasmids of E. coli encode large VirB6-like TraG subunits. These subunits participate in entry exclusion, which prevents nonproductive plasmid transfer between donor cells. TraG’s C-terminal domain extends or is delivered via the T4SS across the donor-donor cell junction, where it binds the entry exclusion protein TraS located in the inner membrane of the paired donor. This interaction might transduce a signal resulting in a conformational change in the T4SS that blocks nonproductive F plasmid transfer to other F-carrying cells (96). (Middle) Rickettsial P-type T4SSs are composed of 4 or more large VirB6 subunits whose hydrophilic domains are unrelated in sequence and which might be surface-displayed for target cell binding or immune evasion (98, 99, 100). (Right) The I-type Dot/Icm system of L. pneumophila secretes the highly hydrophobic DotA (~1,000 residues) to the milieu where it assembles as large ring-shaped complexes. DotA has a canonical signal sequence, which is thought to mediate DotA delivery through the General Secretory Pathway (GSP) across the inner membrane. In the periplasm, DotA is then recruited to the Dot/Icm T4SS for delivery across the outer membrane to the cell exterior (101).