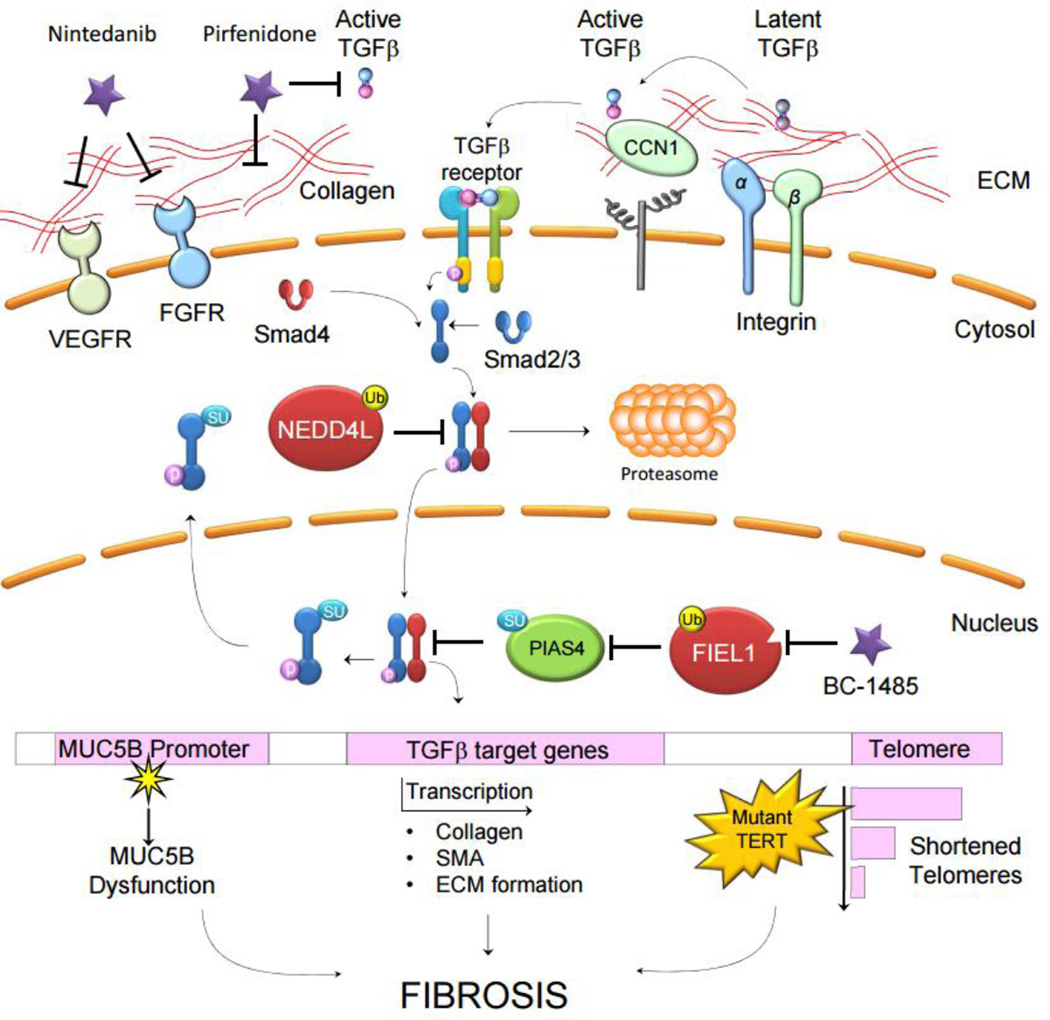
Figure 1.
Membrane proteins such as integrins and secreted proteins in the extracellular matrix (ECM) such as CCN1 interact and facilitate the conversion of latent, ECM-sequestered TGFβ to its active form. Active TGFβ is ligand to the TGFβ receptor on epithelia, fibroblasts, and immune cells. Receptor stimulation causes a signaling cascade via SMAD proteins, leading to the nuclear translocation of a SMAD complex and the transcriptional activation of pro-fibrotic genes. Ubiquitin-E3 ligases, such as NEDD4L, regulate SMAD signaling through proteasome-mediated degradation. Additionally, SUMO-E3 ligases, such as PIAS4, regulate SMAD signaling by forcing its nuclear export. Direct regulators of SMAD signaling are themselves regulated, as FIEL1 catalyzes the degradation of PIAS4. In addition to transcriptional regulation of pro-fibrotic genes and gene products such as collagens, smooth muscle action and fibronectin, genetic abnormalities associate with fibrosis. Mutation within the MUC5B promoter and shortened telomeres caused by telomerase mutations both associate with worsened fibrosis. The current therapeutics Nintedanib and Pirfenidone target growth receptors, ECM formation, and TGFβ. Novel compound BC-1485 targets downstream profibrotic protein FIEL1, which may serve as a new therapeutic approach for the future IPF therapy.