Abstract
Executive functioning (EF), which is considered to govern complex cognition, and verbal memory (VM) are constructs assumed to be related. However, it is not known the magnitude of the association between EF and VM, and how sociodemographic and psychological factors may affect this relationship, including in normal aging. In this study, we assessed different EF and VM parameters, via a battery of neurocognitive/psychological tests, and performed a Canonical Correlation Analysis (CCA) to explore the connection between these constructs, in a sample of middle-aged and older healthy individuals without cognitive impairment (N = 563, 50+ years of age). The analysis revealed a positive and moderate association between EF and VM independently of gender, age, education, global cognitive performance level, and mood. These results confirm that EF presents a significant association with VM performance.
Keywords: executive functioning, verbal memory, aging, canonical correlation analysis, cognition, epidemiology, mental health, psychology
Introduction
Executive functioning (EF) is relevant for cognitive control of behavior. Although models of EF considerably differ, it is consensual that this component governs goal-directed behavior, that is, the control of complex cognition, mainly in non-routine tasks, which require some degree of adaptation to environmental changes. EF has been implicated in the control of processes such as planning, abstraction, reasoning, monitoring, fluency, and other cognitive operations (Lezak, 1995). The study of EF has received great attention in healthy individuals, mainly with respect to its involvement with aspects of cognitive aging (Salthouse, Atkinson, & Berish, 2003). Supporting this thesis, there is robust evidence from neuroanatomical studies showing that EF tasks recruit the activation of frontal areas, particularly the prefrontal cortex (Alvarez & Emory, 2006), which are among the areas known to be particularly affected in the process of aging (Salthouse et al., 2003). Furthermore, it seems evident from the literature that EF affects other cognitive domains, including the association memory, which has been exhaustively explored (Abrahams et al., 2000; Bryson, Whelahan, & Bell, 2001; Proctor, Wilson, Sanchez, & Wesley, 2000; Tremont, Halpert, Javorsky, & Stern, 2000; Vanderploeg, Schinka, & Retzlaff, 1994).
Despite this, the level of the association between these dimensions is not clear, both in healthy individuals as well as in multiple clinical populations. Several reports point to a positive association between EF and verbal memory (VM). For example, Fossati, Amar, Raoux, Ergis, and Allilaire (1999) found a positive association between EF and VM in a schizophrenic patients sample, and Tremont and colleagues (2000) reported that patients with executive dysfunction also performed poorly in VM tasks. Furthermore, individuals with executive dysfunction perform poorly on VM tasks compared with individuals with normal EF (Brooks, Weaver, & Scialfa, 2006). The same pattern was also observed in healthy individuals, where cognitive control levels were positively associated with memory performance (Thomas T. Hills, Mata, Wilke, & Samanez-Larkin, 2013). This may be explained as a reduction of EF capacities which, in turn, conduct to a reduced focus on relevant cues, while switching to distracting ones (Unsworth & Engle, 2007). However, other authors have suggested that this relationship might be mediated by the type of EF tasks. In particular, although subcomponents of EF such as sustained attention and mental tracking were associated with VM, this association was not found with other EF subcomponents such as abstraction, problem solving, and planning (Vanderploeg et al., 1994). Also of note, Bryson and colleagues (2001) verified that EF and VM parameters loaded on separate components in schizophrenic individuals.
The main goal of this work was to analyze the strength of the relation between EF and VM in older individuals without cognitive impairment, comprising all dimensions in one single analysis, through the use of canonical correlation analysis (CCA). Furthermore, we aimed to test whether sociodemographic variables (gender, age, and education), global cognitive performance, and mood status categories play a moderator role in this relation.
Method
Study Sample
A total of 563 healthy individuals (equally distributed with respect to gender) were randomly selected from the Guimarães and Vizela (Portugal) local area health authority registries (health care centers). All participants were community dwellers, being equally distributed between urban and rural areas. The majority of individuals were in the medium socioeconomic stratum (Class III, Graffar measure [Graffar, 1956]) and retired. The cohort was representative of the general Portuguese older population with respect to age (range: 50-89 years, M = 65.23, SD = 9.08, [50-60]: n = 180, 32.0%; [60-70]: n = 192, 34.1%, [70+]: n = 191, 33.9%) and education (median years of formal education = 4 school years, primary education; literacy rate: 99.4%, able to read and write). The distribution of gender for age and education categories is presented in Table 1.
Table 1.
Sample Characteristics.
| Males | Females | Total | |
|---|---|---|---|
| Age | |||
| 50-60 | 86 (47.8%) | 94 (52.2%) | 180 (32.0%) |
| 60-70 | 95 (49.5%) | 97 (50.5%) | 192 (34.1%) |
| 70 or more | 110 (57.6%) | 81 (42.4%) | 191 (33.9%) |
| School years | |||
| 3 or less | 34 (11.7%) | 87 (32.0%) | 121 (21.5%) |
| 4 years | 175 (54.7%) | 145 (45.3%) | 320 (56.8%) |
| 5 or more | 82 (67.2%) | 40 (32.8%) | 122 (21.7%) |
The study was conducted in accordance with the Declaration of Helsinki (59th Amendment) and was approved by the national ethical committee (Comissão Nacional de Proteção de Dados) and by the local ethics review boards (Hospital Escola Braga, Braga; Centro Hospitalar do Alto Ave, Guimarães; and Unidade Local de Saúde do Alto Minho, Viana do Castelo/Ponte de Lima).
The study goals and the neurocognitive assessments were carefully explained to participants. All volunteers provided written informed consent. The primary exclusion criteria included inability to understand informed consent, withdraw from the study, incapacity and/or inability to attend the neuropsychological assessment session(s), and/or diagnosed dementia or neuropsychiatric disorder. All the participants met the proposed threshold for cognitive impairment in individuals with low education levels, based on Mini-Mental State Examination (MMSE) total score (Paulo et al., 2011).
Neurocognitive/Psychological Evaluation
MMSE was assessed as a strategy to identify participants with cognitive impairment. The remaining tests were selected to provide EF and VM profiles. Regarding EF, the following tests were administered: working memory, Digit Span Backward Test (subtest of the Wechsler Adult Intelligence test [WAIS III]); cognitive flexibility and sustained attention, Stroop Color and Word test (Stroop, parameters: interference index for reading and naming); and verbal fluency, Controlled Oral Word Association Test F-A-S (COWAT-FAS, parameters: admissible and non-admissible). With respect to VM, multiple trial verbal learning and memory were assessed through the administration of the Selective Reminding Test (SRT, parameters: consistent long-term retrieval [CLTR], long-term storage [LTS], and delayed recall [DR]). All the above-mentioned tests are robustly validated with respect to the assessment of EF and VM (Santos et al, 2015). The Geriatric Depression Scale (GDS) was administered to assess for (depressive) mood status. A team of trained psychologists conducted the assessments.
Canonical Correlation Analysis
CCA is a multivariate statistical model designed to identify patterns in complex data sets. It allows to study the interrelationships between independent and dependent sets (vectors) of variables. In fact, multivariate statistical procedures can aid in bridging the gap between the theoretical and practical world of behavioral sciences, providing relevant information that cannot be obtained through the use of univariate models (Thompson, 1991). The use of multivariate procedures also limits the probability of committing Type I (experiment wise) errors, that is, the likelihood of finding false statistically significant results (Fan, 1997; Thompson, 1991). The risk of committing Type I errors considerably grows when too many statistical tests are performed on the same variables in a dataset. Furthermore, of biological significance, most human behavior research typically investigates variables that possibly have multiple causes and effects. Therefore, using statistical techniques that are able to handle multiple independent and dependent variables seems appropriate.
To perform CCA, two sets of variables are needed. It is relevant that there is some theoretical meaning behind the construction of the sets, or at least, it should make sense that one group of variables would constitute the independent set of variables, whereas the other would correspond to a dependent set. When these conditions are met, some authors suggest that CCA is the most appropriate and powerful multivariate technique (e.g., Hair, Black, Babin & Anderson, 2010). CCA can be used to address a wide range of objectives: (a) to determine whether two sets of variables are independent of each other or, on the other hand, how they are related; (b) to explain the nature of the relationship between two sets of variables by assessing how each variable contributes to the extracted canonical functions. However, CCA does not assume a dependent versus independent relationship between the two sets of variables. Rather, it analyzes the association between sets: The results would be the same if the X and Y variables were reversed. Technically, the essence of CCA is to form pairs of linear combinations of predictor and criterion variables to maximize the correlation between each pair. Separate sets of coefficients weights are applied to the predictor and criterion variables to form the linear combinations. The canonical correlation itself is the correlation between the linear combinations of predictors and criteria. Canonical correlation can be expressed analytically as follows:
where Y is the set of dependent variables and X the set of independent variables. The sets of original variables are linearly combined to produce pairs of synthetic variables yielding the max bivariate correlation. The procedure of creating synthetic variables is similar to the use of linear equations in multiple regression (Henson, 2002), where beta weights are multiplied with observed scores and then are summed to produce synthetic predicted scores
The synthetic variables obtained in CCA are similar to those produced in other statistical procedures such as principal component analysis (PCA) and discriminant analysis (DA). However, the criteria to obtain those variables differ among the different techniques. In DA, for instance, the original variables are linearly combined to obtain synthetic variables with the objective of maximizing the ratio of between- to within-group variances in such a way that different groups will be maximally differentiated. On the other hand, in PCA, the goal of producing synthetic variables is to account for the max variation of the original variables with the smallest possible number of components.
Statistical Analysis Strategy
The data analysis procedure was structured as follows:
Conversion of all test raw scores into z scores to express all variables in the same scale;
CCA: Two sets of variables were constructed based on EF and VM variables, here considered independent and dependent variables (IV and DV), respectively, for descriptive purposes. EF included Digits Backward, Stroop Words, Stroop Colors, Stroop Words/Colors (WC), and admissible words on COWAT-FAS. VM included scores on SRT test parameters: LTS, CLTR and DR. Three canonical correlations were calculated between the two sets of variables. Canonical variate scores were obtained.
Bivariate correlations between the canonical variables were performed, split by gender, age group (youngest group: 50-60; intermediate group: 60-70; oldest group: 70+), education (0-3 years; 4 years; 5 or more years of formal education), mood status (higher depression, HD; low/no depression, LD; median cutoff), and global cognitive performance (good/poor; classification performed with k-means clustering based on EF and VM canonical scores) categories. Correlation coefficients were then compared using z Fisher Transformation to assess the effects, as given by the following formula:
where r denotes the Pearson correlation coefficient. The canonical correlation between EF and VM sets was assessed through the visual inspection of the scatterplot.
Statistical significance was defined at p<0.05. The R, R Commander (Fox, 2005), CCA (González & Déjean, 2012), YACCA (Yet Another Canonical Correlation Analysis Package [Butts, 2012]), and mvShapiroTest (Gonzales-Estrada & Villasenor, 2013) packages and SPSS v22 (IBM SPSS statistics) were used to perform the statistical analysis.
Results
Descriptive statistics (M and SD) for the cognitive parameters assessed, and the canonical scores for the different group categories (gender, age, education, mood, and global cognitive performance) are provided in Table 2. EF parameters, but not VM parameters, were significantly affected by gender, with scores on these parameters being higher in males. A significant effect of age on EF and VM parameters (all ps < .05, with the exception of COWAT-FAS parameter) was noted, with the youngest group presenting the highest scores on both EF and VM parameters. Participants with less than 3 years of formal education presented lower values on all EF and VM parameters (all ps < .05), compared with those with 4 or more years of education. With respect to mood status, participants with higher depressive status presented lower levels of EF and VM comparing with the lower depressive status group for all EF and VM parameters (all ps < .05). Similarly, the group with poorer global cognitive performance had lower scores on both EF and VM parameters.
Table 2.
Comparison of Cognitive Performance Between Groups.
| Digits back | Stroop words | Stroop colors | Stroop WC | COWAT-FAS | SRT LTS | SRT CLTR | SRT DR | CV1 EF | CV1 VM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | |||||||||||
| 0 = Female (n = 272) | M | 3.72 | 59.47 | 49.04 | 28.97 | 15.07 | 24.95 | 14.43 | 5.5 | 0.37 | 0.01 |
| SD | 1.73 | 17.33 | 11.43 | 10.41 | 8.59 | 11.76 | 10.83 | 2.24 | 0.89 | 1.04 | |
| 1 = Male (n = 291) | M | 4.28 | 68.29 | 48.14 | 29.71 | 18.94 | 24.34 | 13.06 | 5.46 | −0.35 | −0.01 |
| SD | 2.02 | 18.78 | 13.76 | 12.74 | 10.18 | 12.69 | 11.43 | 2.37 | 0.97 | 0.96 | |
| t | −3.528** | −5.777** | 0.841 | −0.756 | −4.865** | 0.596 | 1.456 | 0.24 | 9.176** | 0.338 | |
| d | 0.3 | 0.49 | −0.07 | 0.06 | 0.41 | −0.05 | −0.12 | −0.02 | 0.77 | 0.03 | |
| Age | |||||||||||
| 0 = 50-60 (n = 180) | M | 4.28 | 69.5 | 54.78 | 34.02 | 18.21 | 28.41 | 17.44 | 6.35 | 0.63 | 0.50 |
| SD | 2.01 | 16.42 | 11.51 | 11.61 | 8.92 | 12.16 | 11.78 | 2.12 | 0.61 | 0.65 | |
| 1 = 60-70 (n = 192) | M | 4.03 | 64.32 | 48.15 | 29.58 | 16.66 | 24.88 | 13.57 | 5.45 | 0.08 | −0.01 |
| SD | 1.89 | 19.17 | 12.16 | 12.24 | 9.38 | 11.89 | 10.36 | 2.14 | 1.08 | 1.20 | |
| 2 = 70+ (n = 191) | M | 3.73 | 58.57 | 43.16 | 24.72 | 16.41 | 20.84 | 10.37 | 4.69 | −0.67 | −0.46 |
| SD | 1.8 | 18.5 | 11.67 | 9.11 | 10.45 | 11.57 | 10.25 | 2.35 | 0.78 | 0.82 | |
| F | 3.838* | 16.933** | 45.241** | 32.779** | 1.896 | 18.895** | 19.906** | 26.155** | 109.336** | 50.301** | |
| η2 | 0.01 | 0.06 | 0.14 | 0.11 | 0.01 | 0.06 | 0.07 | 0.09 | 0.28 | 0.15 | |
| Education | |||||||||||
| 0 = 0-3 years (n = 121) | M | 3.12 | 50.15 | 41.64 | 24.79 | 10.66 | 19.42 | 9.11 | 4.33 | −0.18 | −0.44 |
| SD | 1.37 | 16.61 | 10.95 | 10.18 | 6.88 | 9.46 | 7.67 | 2.01 | 1.01 | 1.12 | |
| 1 = 4 years (n = 320) | M | 4.05 | 63.99 | 48.62 | 29.38 | 16.86 | 24.42 | 13.53 | 5.55 | 0.02 | 0.11 |
| SD | 1.89 | 16.23 | 11.83 | 10.73 | 8.44 | 12.04 | 11.16 | 2.15 | 1.02 | 0.96 | |
| 2 = 4+ years (n = 122) | M | 4.77 | 77.89 | 55.35 | 33.80 | 23.98 | 30.37 | 18.80 | 6.44 | 0.12 | 0.14 |
| SD | 2.04 | 15.93 | 12.84 | 13.61 | 10.34 | 12.84 | 12.01 | 2.50 | 0.91 | 0.84 | |
| F | 25.089** | 88.598** | 40.507** | 19.325** | 73.263** | 26.631** | 25.024** | 28.302** | 2.987 | 15.973** | |
| η2 | 0.08 | 0.24 | 0.13 | 0.07 | 0.21 | 0.09 | 0.08 | 0.09 | 0.01 | 0.05 | |
| Mood | |||||||||||
| 0 = LD (n = 291) | M | 4.35 | 69.36 | 50.03 | 30.47 | 19.33 | 26.27 | 14.99 | 5.82 | −0.14 | −0.07 |
| SD | 1.9 | 18.05 | 13.15 | 12.06 | 9.86 | 12.76 | 11.79 | 2.34 | 0.99 | 0.97 | |
| 1 = HD (n = 272) | M | 3.64 | 58.32 | 47.02 | 28.15 | 14.65 | 22.88 | 12.36 | 5.11 | 0.14 | 0.07 |
| SD | 1.85 | 17.5 | 12 | 11.13 | 8.77 | 11.43 | 10.28 | 2.22 | 0.99 | 1.03 | |
| t | 4.450** | 7.356** | 2.827** | 2.363* | 5.932** | 3.312** | 2.811** | 3.715** | 3.398* | 1.647 | |
| d | −0.38 | −0.62 | −0.24 | −0.2 | −0.5 | −0.28 | −0.24 | −0.31 | 0.29 | 0.07 | |
| Cognitive performance | |||||||||||
| 0 = Poor (n = 232) | M | 4.06 | 62.46 | 45.34 | 26.69 | 17.12 | 21.55 | 11.21 | 4.92 | −0.82 | −0.82 |
| SD | 1.92 | 17.77 | 11.89 | 10.05 | 10.37 | 11.80 | 10.59 | 2.37 | 0.82 | 0.81 | |
| 1 = Good (n = 331) | M | 3.97 | 65.13 | 50.85 | 31.22 | 17.04 | 26.80 | 15.48 | 5.87 | 0.57 | 0.58 |
| SD | 1.90 | 19.12 | 12.75 | 12.36 | 9.10 | 12.10 | 11.22 | 2.18 | 0.66 | 0.66 | |
| t | 0.554 | 1.679 | 5.188** | 4.620** | 0.097 | 5.120** | 4.55** | 4.897** | 22.098** | 22.488** | |
| d | 0.05 | −0.07 | −0.44 | −0.39 | 0.01 | −0.43 | −0.38 | −0.41 | −1.87 | −1.9 | |
Note. Stroop WC = Stroop Words/Colors; COWAT-FAS = Controlled Oral Word Association Test F-A-S; SRT = Selective Reminding Test; LTS = long-term storage; CLTR = consistent long-term retrieval; DR = delayed recall; CV1 = canonical variate 1; EF = executive functioning; VM = verbal memory; LD = low depressive mood; HD = high depressive mood.
p < .05. **p < .005 (corrected for multiple comparisons).
Multivariate normality was tested, and significant results were found according to multivariate Shapiro–Wilk test (MVW = 0.968, p < .001). To verify the severity of this violation, multivariate skewness (Sk = 1,023.99) and kurtosis (K = 17.28) were calculated, and the results indicated an asymmetric and platykurtic data distribution. Univariate histograms were performed and evaluated for normality. The minimum values for Sk and K were observed on Stroop Words (Sk = −.099, K = −.403), and the maximum scores were obtained on Digits Backward parameter (Sk = 1.311, K = 4.033) and were considered acceptable considering the following rules-of-thumb: absolute Sk and K values lower than 3.0 and 8.0, respectively (Kline, 2011).
Canonical Correlation Analysis
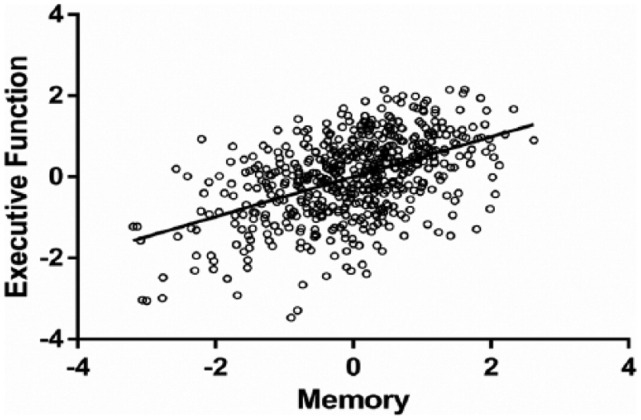
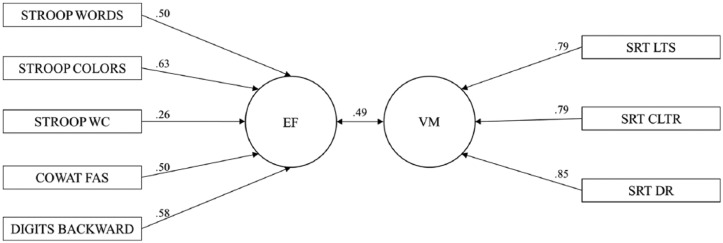
The Barlett’s test for chi-square approximation for the first canonical function (CF) revealed that the sets of variables were not independent of each other, χ2(15) = 161.6, p < .001. With respect to the remaining CFs, the Barlett’s test showed that there was no significant linear combination between EF and VM, neither in the second CF, χ2(8) = 6.75, p = .564, and, consequently, nor in the third, χ2(3) = 0.40, p = .941.1 Each CF represents the maximization of the shared variance between sets, such that the variance not explained by the first function is complemented by the following orthogonal canonical functions. The sets of variables were moderately associated (r = .492) according to the first CF (Figure 1). All the variables of the IV and DV sets presented correlation coefficients above .50, with the exception of Stroop WC, for which a coefficient of .26 was observed. These results highlighted the adequacy of the two dimensions and of the selected parameters (Figure 2). Using the Fisher r to z transformation, the comparison of canonical correlation coefficients with respect to the first CF in subgroup analysis revealed that for gender (r[males] = .526, p < .001; r[females] = .485, p < .001) the difference between correlation coefficients was not significant (z = .59, p = .56). For age categories (r[youngest] = .428, p < .001; r[intermediate group] = .456, p < .001; r[oldest] = .507, p < .001), education (r[0 to 3 years] = .583, p < .001; r[4 years] = .483, p < .001; r[5 or more] = .360), mood status (r[lower mood] = .405, p < .001; r[higher mood] = .574, p < .001), and global cognitive performance (r[good performers] = .463, p < .001; r[poor performers] = .539, p < .001) all the differences given by z were not statistically significant, meaning that none of these variables can be considered moderators of the association between EF and VM.
Figure 1.
Scatterplot representing the association between EF and VM variates.
Note. EF = executive functioning; VM = verbal memory.
Figure 2.
Loadings of each parameter on EF/VM dimension.
Note. EF = executive functioning; VM = verbal memory.
Discussion
In a cohort of healthy individuals, we analyzed the relationship between EF and VM parameters. Using CCA, we were able to detect a modest, but significantly positive, association between these two parameters. Furthermore, it is of note that this relation seems to be independent of sociodemographic variables (such as gender, age, or school years), mood status (as measured by GDS), and, interestingly, global cognitive performance (that is, stronger or poorer global cognitive performance). To the best of our knowledge, only one study (Duff, Schoenberg, Scott, & Adams, 2005) has examined the association between EF and VM through the use of CCA. However, the study analyzed the relationship between EF and VM in a clinical sample with suspected neurological and psychiatric conditions. Since this technique is considered appropriate when dealing with sets of variables, it is somehow surprising the lack of studies addressing this topic with this statistical method. In this context, although the interplay between EF and VM is well established, the relationship between EF and VM is typically analyzed between single dimensions of each construct. By using this multivariate technique, we are able to assess this relationship taking into consideration the relevance of all components of each dimension.
Herein, although the canonical correlation between the sets of variables that comprise EF and VM is relatively modest, it deserves some reflection considering its significant and stable association. These results seem to corroborate a previous theory regarding age-related dynamics involved in memory tasks – the cue-maintenance hypothesis (Unsworth & Engle, 2007). This view postulates that memory is a dynamic process in which individuals rely on relevant cues to access learned materials. Thus, in accordance with previous suggestions (Hills, Todd, & Goldstone, 2010), it is plausible to hypothesize that reduced EF performance – reduced cognitive control, particularly – may conduct to a decreased ability to ignore distracting cues, while attending to relevant ones. This may be translated in a reduced VM capacity.Possible explanations may stem from neuroanatomical evidence. The prefrontal cortex, an area that is known to be importantly involved in EF tasks, seems to play a supervisory role in the encoding and retrieval of VMs (Nyberg & Cabeza, 2000). Moreover, recent evidences have suggested that an increased thinning in frontal and cingulate cortices in mild cognitive impairment individuals (a condition characterized by executive dysfunction) was also related with worse performance on memory tasks, which is beyond the robust dependence of memory deficits on hippocampal atrophy (Chang et al., 2010) and other medial temporal structures (Brown & Aggleton, 2001). In line with these findings, it is possible that the present results, pointing to a stable association between EF and VM, may derive from the impact that brain structures involved in EF processing have on the performance in VM tasks. However, it is also of relevance that only near 25% of the variance is shared by canonical scores of EF and VM, which highlights an independence between these two constructs. As abovementioned, the memory of verbal stimuli is not strictly dependent on the hippocampus, with other structures from the temporal lobe (particularly from the left hemisphere) also playing a relevant role. In fact, the hippocampus, parahippocampal gyrus and perirhinal cortex seem to display dissociable (albeit complementary) roles during verbal encoding. Particularly, it has been suggested that the perirhinal cortex and the hippocampus work in an integrated fashion during the encoding of verbal information (Brown & Aggleton, 2001). On the other hand, parahippocampal activations have been noted in a functional magnetic resonance imaging (fMRI) study during the encoding of the first elements on a VM task (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998). Therefore, it can possibly be argued that the variety of brain structures implicated in memory encoding and retrieval may contribute to reduce the strength of association between these dimensions. Here, the fact that the EF/VM relationship was not affected by sociodemographic variables (gender, age, or education), mood, or global cognitive performance enhances the possibility that there are some stable neurobiological mechanisms underlying the performance on both EF and VM tasks.
Authors have suggested that EF underlies other complex cognitive tasks, namely, episodic memory (McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010). It is proposed that age-related declines in some aspects of EF (such as executive attention) underlie the deterioration of episodic memory observed during the process of aging. In fact, neuroanatomical explanations have been put forward in support of this hypothesis, namely, the interplay between prefrontal cortex and medial temporal lobe structures, most likely mediated by thalamic pathways (Ketz, Jensen, & O’Reilly, 2015). Moreover, it was suggested that prefrontal cortex plays an important role in the use of the context to guide the retrieval of memories, and that the memory consolidation depends on the integrity of temporal lobe structures, particularly the hippocampus (Preston & Eichenbaum, 2013). Thus, it is possible that the absence of statistically significant differences in the associations between the different aspects reported here are due to the fact that alterations in one component (EF) appear to underlie alterations in the other (VM), independently of sociodemographic aspects.
Some study limitations should be addressed. Although the focus of the analysis is on VM, episodic memory is not limited to this component. Therefore, the results should be interpreted with caution when generalizing the association between EF and episodic memory to other episodic memory parameters further than VM. Our sample was restricted to adults with 50 or more years of age. It would be interesting to assess the relationship herein reported in younger individuals. In fact, it is well known that several aspects of cognitive performance are known to decrease with age. Indeed, several aspects, such as processing speed, reasoning, and memory are affected with increased age, being suggested that these age-related differences start early in adulthood, probably even already in the 20s (Salthouse, 2004). Thus, it would be relevant to explore the association between the constructs here analyzed in other developmental phases. Finally, the definition of cognitive performance categories based on the results of canonical scores for both dimensions can be questioned. However, this strategy was considered to be relevant because it proved that the relationship does not differ significantly between individuals with higher and lower scores on the cognitive parameters assessed, adding as well an interesting aspect in that associations may be tied in regardless of performance level.
In sum, our results corroborate and extend on the reported evidences from neuroanatomical studies, in which EF significantly affected VM performance, with this apparent dependence being verified independently of sociodemographic and neurocognitive/psychological characteristics such as gender, age, mood, and global cognitive performance. In addition, we highlight the relevance of the use of the analytical procedure here presented to study the relationship between other cognitive or psychological domains.
Acknowledgments
The authors are thankful to all colleagues who aided in data collection and to all study participants.
The number of canonical functions obtained in the canonical correlation analysis equals the number of variables of the set with lower number of variables: In this case, there were produced three canonical functions, corresponding to the number of variables of the verbal memory (VM) set.
Footnotes
Authors’ Note: P.S.M. and N.C.S. contributed equally in the authorship and should be listed as co-authors.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the European Commission (FP7): “SwitchBox” (Contract HEALTH-F2-2010-259772) and co-financed by the Portuguese North Regional Operational Program (ON.2–O Novo Norte) under the National Strategic Reference Framework (QREN), through the European Regional Development Fund (FEDER). P.S.M. is supported by a “MyHealth” project (Contract DoIT-13853) doctoral fellowship and N.C.S. by a “SwitchBox” project post-doctoral fellowship.
References
- Abrahams S., Leigh P. N., Harvey A., Vythelingum G. N., Grise D., Goldstein L. H. (2000). Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia, 38, 734-747. [DOI] [PubMed] [Google Scholar]
- Alvarez J. A., Emory E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16, 17-42. doi: 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Brewer J. B., Zhao Z., Desmond J. E., Glover G. H., Gabrieli J. D. (1998). Making memories: Brain activity that predicts how well visual experience will be remembered. Science, 281, 1185-1187. [DOI] [PubMed] [Google Scholar]
- Brooks B. L., Weaver L. E., Scialfa C. T. (2006). Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? Clinical Neuropsychologist, 20, 230-242. doi: 10.1080/13854040590947461 [DOI] [PubMed] [Google Scholar]
- Brown M. W., Aggleton J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51-61. [DOI] [PubMed] [Google Scholar]
- Bryson G., Whelahan H. A., Bell M. (2001). Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Research, 102, 29-37. [DOI] [PubMed] [Google Scholar]
- Butts C. T. (2012). yacca: Yet Another Canonical Correlation Analysis Package. R Package Version 1.1. Retrieved from http://CRAN.R-project.org/package=yacca
- Chang Y. L., Jacobson M. W., Fennema-Notestine C., Hagler D. J., Jr., Jennings R. G., Dale A. M., McEvoy L. K. (2010). Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cerebral Cortex, 20, 1305-1313. doi: 10.1093/cercor/bhp192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Schoenberg M. R., Scott J. G., Adams R. L. (2005). The relationship between executive functioning and verbal and visual learning and memory. Archives of Clinical Neuropsychology, 20, 111-122. doi: 10.1016/j.acn.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Fan X. (1997). Canonical correlation analysis and structural equation modeling: What do they have in common? Structural Equation Modeling: A Multidisciplinary Journal, 4, 65-79. doi: 10.1080/10705519709540060 [DOI] [Google Scholar]
- Fossati P., Amar G., Raoux N., Ergis A. M., Allilaire J. F. (1999). Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research, 89, 171-187. [DOI] [PubMed] [Google Scholar]
- Fox J. (2005). The R commander: A basic statistics graphical user interface to R. Journal of Statistical Software, 14(9), 1-42. [Google Scholar]
- Gonzales-Estrada E., Villasenor J. (2013). Generalized Shapiro-Wilk test for multivariate normality (Version 1.0). Retrieved from http://CRAN.R-project.org/package=mvShapiroTest
- González I., Déjean S. (2012). CCA: Canonical correlation analysis. R Package Version 1.2. Retrieved from http://CRAN.R-project.org/package=CCA
- Graffar M. (1956). Une méthode de classification sociale d’échantillons de population [A method of social classification of population]. Courrier, 6, 455-459. [Google Scholar]
- Hair J. F., Black B., Babin B., Anderson R. E. (2010). Multivariate data analysis (7th ed.). Upper saddle River, NJ: Prentice Hall. [Google Scholar]
- Henson R. (2002, April). The logic and interpretation of structure coefficients in multivariate general linear model analyses. Paper presented at the Annual Meeting of the American Educational Research Association, New Orleans, LA. [Google Scholar]
- Hills T. T., Mata R., Wilke A., Samanez-Larkin G. R. (2013). Mechanisms of age-related decline in memory search across the adult life span. Developmental psychology, 49(12), 2396-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T. T., Todd P. M., Goldstone R. L. (2010). The central executive as a search process: Priming exploration and exploitation across domains. Journal of Experimental Psychology: General, 139(4), 590-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N. A., Jensen O., O’Reilly R. C. (2015). Thalamic pathways underlying prefrontal cortex–medial temporal lobe oscillatory interactions. Trends in Neurosciences, 38, 3-12. doi: 10.1016/j.tins.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Kline R. B. (2011). Principles and practice of structural equation modeling. New York, NY: Guilford Press. [Google Scholar]
- Lezak M. D. (1995). Neuropsychological assessment. New York, NY: Oxford University Press. [Google Scholar]
- McCabe D. P., Roediger H. L., McDaniel M. A., Balota D. A., Hambrick D. Z. (2010). The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology, 24, 222-243. doi: 10.1037/a0017619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Cabeza R. (2000). Brain imaging of memory. In Tulving E., Craik F. (Eds.), The Oxford handbook of memory (pp. 501-519). New York, NY: Oxford University Press. [Google Scholar]
- Paulo A. C., Sampaio A., Santos N. C., Costa P. S., Cunha P., Zihl J., . . . Sousa N. (2011). Patterns of cognitive performance in healthy ageing in Northern Portugal: A cross-sectional analysis. PLoS ONE, 6(9), e24553. doi: 10.1371/journal.pone.0024553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A. R., Eichenbaum H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23, R764-R773. doi: 10.1016/j.cub.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor A., Wilson B., Sanchez C., Wesley E. (2000). Executive function and verbal working memory in adolescents with closed head injury (CHI). Brain Injury, 14, 633-647. [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2004). What and when of cognitive aging. Current Directions in Psychological Science, 13, 140-144. doi: 10.1111/j.0963-7214.2004.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A., Atkinson T. M., Berish D. E. (2003). Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General, 132, 566-594. doi: 10.1037/0096-3445.132.4.566 [DOI] [PubMed] [Google Scholar]
- Santos N. C., Costa P. S., Amorim L., Moreira P. S., Cunha P., Cotter J., Sousa N. (2015). Exploring the factor structure of neurocognitive measures in older individuals. PloS one, 10(4), e0124229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. L. (1991). A unified approach to rank tests for multivariate and repeated measures designs. Journal of the American Statistical Association, 86, 410-419. doi: 10.1080/01621459.1991.10475058 [DOI] [Google Scholar]
- Tremont G., Halpert S., Javorsky D. J., Stern R. A. (2000). Differential impact of executive dysfunction on verbal list learning and story recall. Clinical Neuropsychologist, 14, 295-302. doi: 10.1076/1385-4046(200008)14:3;1-p;ft295 [DOI] [PubMed] [Google Scholar]
- Unsworth N., Engle R. W. (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological review, 114(1), 104-132. [DOI] [PubMed] [Google Scholar]
- Vanderploeg R. D., Schinka J. A., Retzlaff P. (1994). Relationships between measures of auditory verbal learning and executive functioning. Journal of Clinical and Experimental Neuropsychology, 16, 243-252. doi: 10.1080/01688639408402635 [DOI] [PubMed] [Google Scholar]