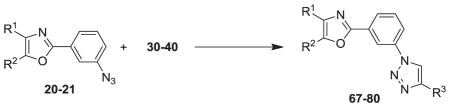
Table 3.
Activities of 2-(3-triazolylphenyl)-4,5-Diphenyloxazole Click Products 67-80
| ||||
|---|---|---|---|---|
| Compound (Yield%)1 | IC50 (μM) | S. gordonii growth inhibition (%) | P. gingivalis growth inhibition (%) | Concentration (μM) |
| 67, R1, R2=4-Methoxyphenyl, R3=3-Pyridyl (89) | 57.7 | |||
| 68, R1, R2=4-Methoxyphenyl, R3=3-Fluorophenyl (97) | 5.9 | −9.1 | −2.6 | 40 |
| 69, R1, R2=4-Methoxyphenyl, R3=4-Fluorophenyl (86) | 53.0 | −22.1 | −14.6 | 40 |
| 70, R1, R2=4-Methoxyphenyl, R3=2-Methoxyphenyl (83) | 15.4 | 0.8 | −6.2 | 40 |
| 71, R1, R2=4-Methoxyphenyl, R3=2-(Trifluoromethyl)phenyl (95) | 27.0 | −9.7 | −1.6 | 40 |
| 72, R1, R2=4-Methoxyphenyl, R3=4-(n-Pentyl)phenyl (99) | >60 | |||
| 73, R1, R2=4-Methoxyphenyl, R3=6-Methoxynaphthalene-2-yl (77) | 13.0 | −1.3 | 3.0 | 20 |
| 74, R1, R2=4-Methoxyphenyl, R3=4-Phenoxyphenyl (89) | >60 | |||
| 75, R1, R2=4-Methoxyphenyl, R3=3,5-di-(Trifluoromethyl)phenyl (95) | 47.4 | |||
| 76, R1, R2=4-Bromophenyl, R3=3-Pyridyl (72) | 31.0 | |||
| 77, R1, R2=4-Bromophenyl, R3=3-Fluorophenyl (85) | 5.0 | −5.7 | −4.3 | 5 |
| 78, R1, R2=4-Bromophenyl, R3=2-Methoxyphenyl (71) | 15.0 | 10.5 | 4.3 | 40 |
| 79, R1, R2=4-Bromophenyl, R3=2-(Trifluoromethyl)phenyl (90) | 15.2 | 2.0 | −0.6 | 40 |
| 80, R1, R2=4-Bromophenyl, R3=6-Methoxynaphthalene-2-yl (91) | 20.3 | |||
Yields are for isolated pure compounds.
