Figure S1.
Isolation of Translational Decoding Complexes for Cryo-EM, Related to Figure 1
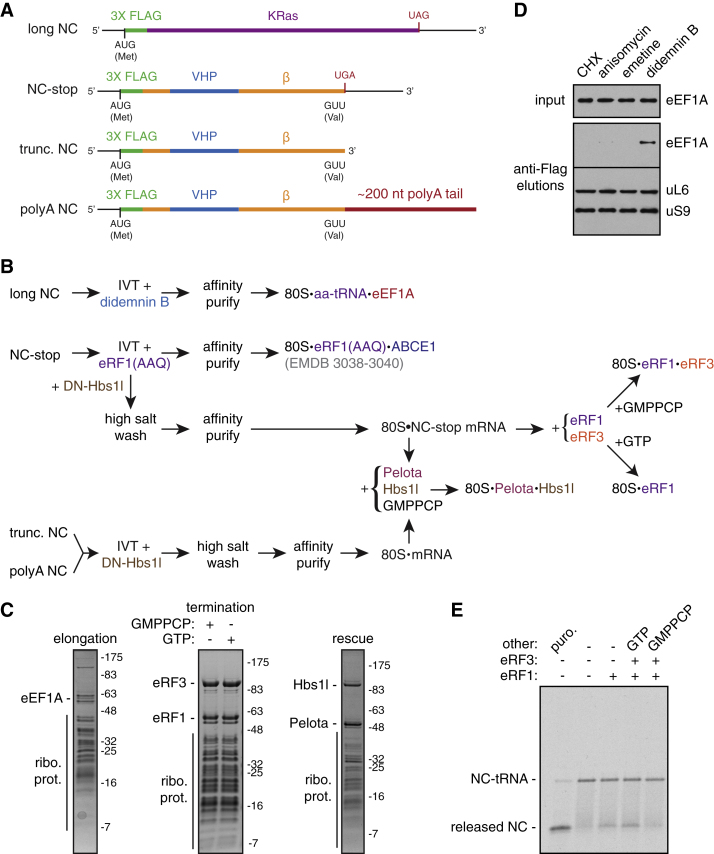
(A) Schematic of the mRNA constructs used for in vitro translation and isolation of ribosome-nascent chain complexes (RNCs). The start codon (AUG), stop codon (UAG or UGA), and coding regions for the 3X Flag tag (green), the autonomously-folding villin headpiece (VHP) domain (blue), the cytosolic portion of Sec61β (orange), and KRas (purple) are indicated.
(B) Experimental strategies for isolating the indicated RNCs from in vitro translation (IVT) reactions.
(C) SDS-PAGE and Coomassie staining of isolated RNCs representing the elongation complex (80S⋅aa-tRNA⋅eEF1A); pre-accommodated (80S⋅eRF1⋅eRF3) or accommodated (80S⋅eRF1) termination complexes; and rescue complex (80S⋅Pelota⋅Hbs1l) reconstituted with a truncated mRNA (see panel A). Copurified, exogenously-added, and ribosomal (ribo. prot.) proteins are indicated.
(D) The long NC construct (see panel A) was translated in vitro in rabbit reticulocyte lysate (RRL) with the indicated translational inhibitors added at the following concentrations: 50 μg/mL cycloheximide (CHX), 10 μM anisomycin, 200 μM emetine, and 50 μM didemnin B. The translation reactions were affinity purified via the 3X Flag tag on the nascent chain. The elutions and inputs were analyzed by SDS-PAGE and immunoblotting for the indicated proteins, revealing that didemnin B specifically traps eEF1A on the isolated RNCs.
(E) The NC-stop construct was translated in vitro in RRL in the presence of 35S-methionine and mutant eRF1(AAQ) to trap RNCs with the UGA stop codon in the A site. The RNCs were isolated under high salt conditions and subjected to affinity purification via the 3X Flag tag on the nascent chain. The isolated RNCs were incubated with 1 mM puromycin or recombinant wild-type eRF1, wild-type eRF3, and 0.5 mM GMPPCP or GTP as indicated, and then directly analyzed by SDS-PAGE and autoradiography. The bands corresponding to ribosome-associated nascent chain-tRNA (NC-tRNA) and released nascent chains (NC) are indicated. This demonstrates the functionality of the components of the reconstituted termination complex in mediating the release of the nascent chain, which is inhibited by the nonhydrolyzable GTP analog, GMPPCP.