Abstract
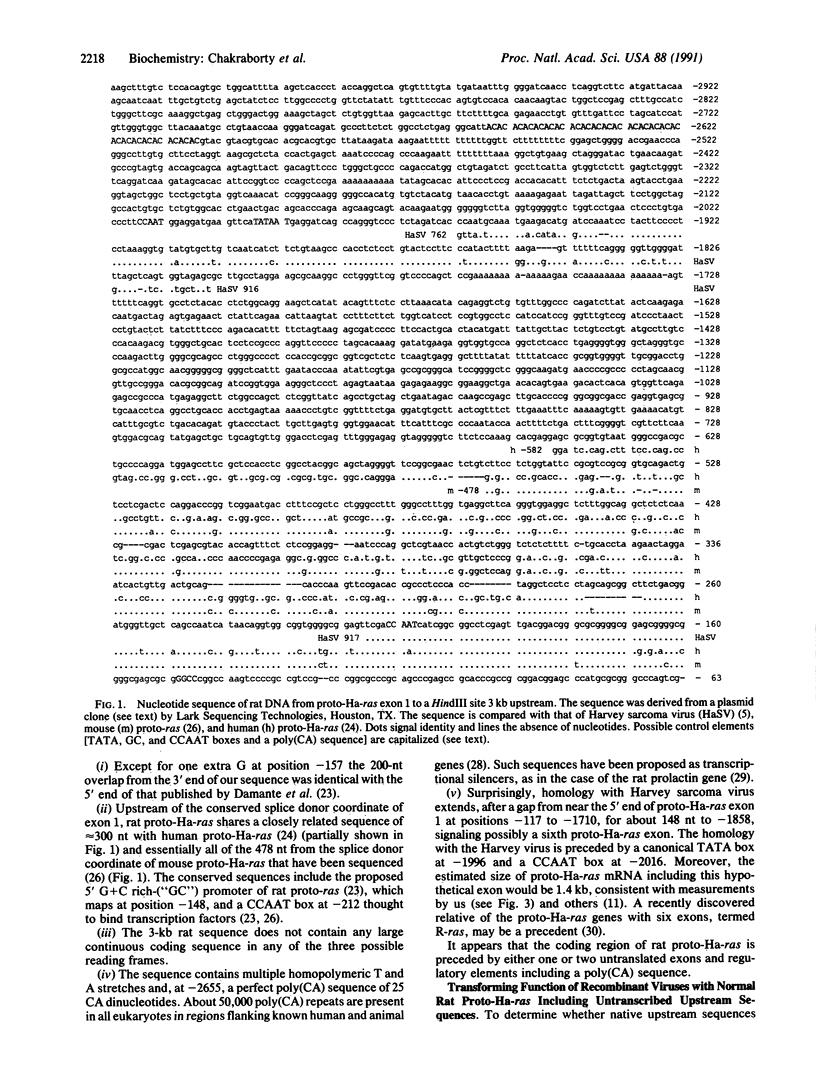
Based on transfection into cells in culture or natural transduction into retroviruses, proto-ras genes seem to derive transforming function either from heterologous promoters or from point mutations. Here we ask how such different events could achieve the same results. To identify homologous regulatory elements, about 3 kilobases of rat DNA upstream of the first untranslated proto-Ha-ras exon was sequenced. Surprisingly, the sequence shares at -1858 a homology of 148 nucleotides with Harvey (Ha) sarcoma virus, 5' of viral ras, signaling possibly a second untranslated proto-Ha-ras exon. In addition the sequence contains a perfect repeat of 25 CA dinucleotides at -2655. A retroviral promoter, even from upstream of the poly(CA), conferred transforming function on proto-Ha-ras and increased transcription greater than 100-fold compared with that of unrearranged proto-ras. Point mutations were not necessary for transforming function of rat and human proto-Ha-ras genes with retroviral promoters but did enhance it greater than 10-fold. A unifying hypothesis proposes that proto-ras genes depend on high expression from heterologous promoters or enhancers for transforming function, which is modulated by ras point mutations. The hypothesis makes two testable predictions. (i) Unrearranged proto-ras genes with point mutations, which occur in some cancers, have no transforming function. Indeed, tumors with mutated proto-ras genes, even those that also lack hypothetical tumor-suppressor genes, are indistinguishable from counterparts with normal proto-ras genes. (ii) Proto-ras genes in transfected cells derive transforming function from heterologous promoters or enhancers acquired via illegitimate recombination from vector DNAs and particularly from viral helper genes that must be cotransfected for transformation of primary cells. Indeed, expression of exogenous proto-ras genes in cells transformed by transfection is as high as for viral ras genes and is much higher than in the cells of origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Le Strange R., Oliff A. I., Furth M. E., Old L. J. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984 Mar 1;308(5954):69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Brown K., Bailleul B., Ramsden M., Fee F., Krumlauf R., Balmain A. Isolation and characterization of the 5' flanking region of the mouse c-Harvey-ras gene. Mol Carcinog. 1988;1(3):161–170. doi: 10.1002/mc.2940010304. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Morgan P. L., Lee E. J., Pirollo K. F., White E. A., Patrick D. H., Tsichlis P. N. Pathogenicity of retroviruses containing either the normal human c-Ha-ras1 gene or its mutated form derived from the bladder carcinoma EJ/T24 cell line. J Exp Pathol. 1985 Fall;2(3):177–189. [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Recombinant BALB and Harvey sarcoma viruses with normal proto-ras-coding regions transform embryo cells in culture and cause tumors in mice. J Virol. 1989 Mar;63(3):1377–1383. doi: 10.1128/jvi.63.3.1377-1383.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damante G., Filetti S., Rapoport B. Nucleotide sequence and characterization of the 5' flanking region of the rat Ha-ras protooncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):774–778. doi: 10.1073/pnas.84.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Activated proto-onc genes: sufficient or necessary for cancer? Science. 1985 May 10;228(4700):669–677. doi: 10.1126/science.3992240. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Cancer genes: rare recombinants instead of activated oncogenes (a review). Proc Natl Acad Sci U S A. 1987 Apr;84(8):2117–2124. doi: 10.1073/pnas.84.8.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Zhou R. P., Goodrich D. Cancer genes by illegitimate recombination. Ann N Y Acad Sci. 1989;567:259–273. doi: 10.1111/j.1749-6632.1989.tb16477.x. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., O'Neill R. R., Rutledge R. A., Theodore T. S., Martin M. A., Ruscetti S. K., Austin J. B., Hartley J. W. Biologic and molecular characterization of two newly isolated ras-containing murine leukemia viruses. J Virol. 1987 Jul;61(7):2109–2119. doi: 10.1128/jvi.61.7.2109-2119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Evidence that retroviral transduction is mediated by DNA not by RNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3604–3608. doi: 10.1073/pnas.87.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral transduction of oncogenic sequences involves viral DNA instead of RNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3733–3737. doi: 10.1073/pnas.85.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Cairns J. The secrets of cancer. Nature. 1982 Nov 11;300(5888):104–105. doi: 10.1038/300104a0. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Capon D. J., Delwart E., Sakaguchi A. Y., Naylor S. L., Goeddel D. V. Structure of the human and murine R-ras genes, novel genes closely related to ras proto-oncogenes. Cell. 1987 Jan 16;48(1):137–146. doi: 10.1016/0092-8674(87)90364-3. [DOI] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. The polymerase chain reaction and cancer diagnosis. Cancer Cells. 1989 Oct;1(2):56–61. [PubMed] [Google Scholar]
- Naylor L. H., Clark E. M. d(TG)n.d(CA)n sequences upstream of the rat prolactin gene form Z-DNA and inhibit gene transcription. Nucleic Acids Res. 1990 Mar 25;18(6):1595–1601. doi: 10.1093/nar/18.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Gazit A., Arnstein P., Aaronson S. A. Generation of fibrosarcomas in vivo by a retrovirus that expresses the normal B chain of platelet-derived growth factor and mimics the alternative splice pattern of the v-sis oncogene. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2693–2697. doi: 10.1073/pnas.86.8.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Norman G. L., Heidecker G. Nucleotide sequence of the Rasheed rat sarcoma virus oncogene: new mutations. Science. 1983 Jul 8;221(4606):155–157. doi: 10.1126/science.6344220. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Lipman D., Andersen P. R., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of the BALB/c murine sarcoma virus transforming gene. J Virol. 1985 Mar;53(3):984–987. doi: 10.1128/jvi.53.3.984-987.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P. Nucleotide sequence analysis of the T24 human bladder carcinoma oncogene. Science. 1983 Jun 3;220(4601):1061–1063. doi: 10.1126/science.6844927. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Maronpot R. R., Anderson M. W., Aaronson S. A. Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):33–37. doi: 10.1073/pnas.83.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P. Proto-oncogenes II. Med Hypotheses. 1988 Dec;27(4):289–290. doi: 10.1016/0306-9877(88)90009-6. [DOI] [PubMed] [Google Scholar]
- Rosen P. The significance of proto-oncogenes in carcinogenesis. Med Hypotheses. 1987 Jan;22(1):23–26. doi: 10.1016/0306-9877(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Transforming collaborations between ras and nuclear oncogenes. Cancer Cells. 1990 Aug-Sep;2(8-9):258–268. [PubMed] [Google Scholar]
- Ruta M., Wolford R., Dhar R., Defeo-Jones D., Ellis R. W., Scolnick E. M. Nucleotide sequence of the two rat cellular rasH genes. Mol Cell Biol. 1986 May;6(5):1706–1710. doi: 10.1128/mcb.6.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985 Jul 11;316(6024):160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Identifying tumor suppressor genes in human colorectal cancer. Science. 1990 Jan 5;247(4938):12–13. doi: 10.1126/science.2403692. [DOI] [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Weinberg R. A. Analysis of viral and somatic activations of the cHa-ras gene. J Virol. 1985 Jan;53(1):260–265. doi: 10.1128/jvi.53.1.260-265.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. We still don't understand cancer. Nature. 1983 Apr 21;302(5910):656–656. doi: 10.1038/302656a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Vass W. C., Lowy D. R., Tambourin P. E. Harvey murine sarcoma virus: influences of coding and noncoding sequences on cell transformation in vitro and oncogenicity in vivo. J Virol. 1989 Mar;63(3):1384–1392. doi: 10.1128/jvi.63.3.1384-1392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Zhou R. P., Duesberg P. H. Avian proto-myc genes promoted by defective or nondefective retroviruses are single-hit transforming genes in primary cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7721–7725. doi: 10.1073/pnas.86.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]





