Abstract
Ultrasound is an essential modality within musculoskeletal imaging, with the recent addition of elastography. The elastic properties of tissues are different from the acoustic impedance used to create B mode imaging and the flow properties used within Doppler imaging, hence elastography provides a different form of tissue assessment. The current role of ultrasound elastography in the musculoskeletal system will be reviewed, in particular with reference to muscles, tendons, ligaments, joints and soft tissue tumours. The different ultrasound elastography methods currently available will be described, in particular strain elastography and shear wave elastography. Future directions of ultrasound elastography in the musculoskeletal system will also be discussed.
Keywords: Ultrasound, Elastography, Musculoskeletal system
Core tip: This review article describes the different techniques of ultrasound elastography and their current and future role in the musculoskeletal system.
INTRODUCTION
Ultrasound is an essential modality within medical imaging, predominantly for assessing soft tissues. Recently, the additional tool of ultrasound elastography has become commercially available for further assessment of tissues, in addition to the standard B-mode and Doppler imaging. The elastic properties of tissues are different from the acoustic impedance used to create B mode imaging and the flow properties used within Doppler imaging, hence elastography provides a different form of tissue assessment and possibly showing pathology before it can be detected on B mode imaging. This may be of particular use in the musculoskeletal system where there is a wide spectrum of tissue specialisation. Elastography assesses the strain (stiffness) of these tissues in response to stress, through a variety of different methods which will be discussed.
Throughout history, the stiffness of tissues has been used as a marker of disease, through palpation. Generally, malignant tissues are stiffer or harder than benign tissues, a feature which can be distinguished through direct palpation, corresponding to manual compression. This concept has been extended within the field of ultrasound, with maps of tissue stiffness generated alongside anatomical images.
PRINCIPLES OF ELASTOGRAPHY
The term elastography was described by Ophir et al[1] as a method of portraying the strain properties of biological tissue. The strain of a tissue is its response to an applied stress, or pressure, with both longitudinal and shear components. A longitudinal strain occurs when a tissue is compressed or stretched, whilst a shear strain is the response to angular forces, such as twisting. Biological tissues have both viscous (liquid-like) and elastic (solid-like) properties, with a complex interplay between the two given the non-uniform nature of biological tissues. When a stress is applied to fluids, the pressure is the same in all directions, hence shear strain and shear waves do not exist in pure fluids. The Elastic modulus of a material gives an indication of how it responds to a change in applied stress and is defined as the slope of the stress-strain curve. The elastic modulus can be described as either Young’s modulus (E) (compressive stress/compressive strain) or the shear modulus (G) (shear stress/shear strain). The bulk modulus is a three dimensional extension of Young’s modulus and describes volumetric stress over volumetric strain[2]. Young’s modulus and shear modulus are the most applicable to biological tissues, with an approximation between them described as E ≅ 3G. The elastic modulus of a tissue is inversely proportional to the strain, i.e., the greater the elastic modulus, the less the tissue strain.
In medical usage, elastography requires the application of a mechanical stress to the tissues and then measurement of the displacement before and immediately after the stress as an estimate of the strain[3]. Soft tissues in the body have a high water content and are virtually incompressible, thus sophisticated equipment is necessary to detect small tissue displacements. There are currently two main elastography methods in general clinical usage, namely compression/strain elastography and shear wave elastography.
Strain elastography
In strain, or compression elastography, a force (i.e., stress) is applied from the transducer by repetitive manual pressure and the displacement (strain) is calculated from the return velocities of the tissues with respect to time. Motion intrinsic to the subject can also be used as the stress generator, such as aortic pulsation, however this is less useful in the musculoskeletal system where more superficial structures are of interest.
Measuring the displacement (strain) of the tissues secondary to an applied force (stress) gives a qualitative map of the elastic modulus distribution, termed an elastogram. This elastogram is colour-coded and often super-imposed on a grey-scale B mode image for anatomical localisation. True quantitative measures cannot be taken from this elastogram, as the applied force is unknown. A semi-quantitative evaluation, however, can be determined from the ratio of the displacement of the tissue of interest and an adjacent structure, such as subcutaneous fat.
Strain elastography has many potential disadvantages, including the variability in the pressure applied to the tissue. This can be partly compensated for by a graphical representation of the adequacy of the compression, however, the potential for inter-observer and intra-observer variation remains[4]. At least three cycles of compression and decompression have been recommended for optimum assessment[5], however, extensive preload of the tissues through repeated compressions can alter tissue elasticity. Thus, enough compression/decompression cycles must performed to obtain a representative assessment, however, excessive compression may adversely affect the resulting elastograms through pre-loading the tissues. Correct probe alignment is required to compensate for potential out of plane compression of the tissue and anisotropy. Compression along the longitudinal axis of the region of interest has been shown to be optimal as compression in the transverse plane gives rise to artefacts at the medial and lateral sides of the image and out-of-plane movement. Constraints from bony anatomy, such as around the ankle, can also make uniform compression difficult across the region of interest, in particular in the musculoskeletal system.
Shear wave elastography
Shear wave elastography applies a vibration to tissues through a focussed ultrasound pulse, generated by the transducer. This deposition of energy within the tissues creates transverse waves, or shear waves, which are perpendicular to the push pulse. The shear wave velocities can be measured from Doppler frequency modulation of simultaneously transmitted probing ultrasound waves. Young’s modulus can then be estimated as a function of the shear wave velocity. The stiffer the tissue is (the less compliant to shear forces), the faster the propagated shear waves within it[6].
Although shear wave elastography is likely to be more reproducible than strain elastography owing to the standardised applied stress, it still has limitations. Shear waves are attenuated at depth and thus very deep tissues (> 9 cm from the skin) cannot be assessed. Conversely, an adequate depth of tissue is required in order to generate shear waves, hence very superficial structures are difficult to assess. This can partly be compensated for by using a gel stand-off on the patient. Shear waves are not generated within fluids, thus cystic structures cannot be adequately analysed[7]. The size of the region of interest may also potentially affect the shear wave measurements. Similar to strain elastography, the amount of probe pressure applied to the skin has the potential to affect the tissue elasticity, through pre-loading the tissues, as shown in a study assessing muscle elastic properties with shear wave elastography and varying technical parameters[8]. Assessment of a tissue in the longitudinal vs transverse plane may potentially affect the shear wave measurements owing to anisotropy, similar to strain elastography. The acoustic radiation force impulse required to generate shear waves deposits energy in the tissues, manifest partly as heating. This should be considered with repeated measurements, as the heating effect may adversely alter the measurements or potentially cause tissue damage. Commercially available systems have an in-built cooling delay in the order of a few seconds, to reduce any potential heating, however, prolonged usage may still heat the tissues and alter the properties of the generated shear waves.
Measured values of shear waves are given as either a velocity in m/s or a stiffness in kPa The elasticity expressed in kPa can be approximated into shear wave velocity using the equation E = 3rc2, where E is Young modulus, r is density (estimated at 1000 kg/m3), and c is the speed of sound[9]. This method of converting the values is useful in isotropic tissues, such as the breast, however, it may not be applicable to tendons and muscles which are anisotropic, with potential for faster propagation of shear waves along the longitudinal axis of the tendon[10]. Thus, caution must be used when comparing values quoted in kPa with m/s as they may not be directly comparable.
A study assessing the differences between two commercially available systems for shear wave elastography has shown that the results in assessing liver stiffness are broadly similar, however, the measurements in kPa vs m/s are not directly interchangeable, thus, the machines should be kept constant if temporal follow-up is required[11]. Reference standards for shear wave elastography have been determined in many tissues, including the thyroid, submandibular glands, masseter, gastrocnemius and supraspinatus muscles and the Achilles tendon[12], however these should be used with caution given the potential for poor reproducibility between vendor equipment.
Transient elastography
Another form of elastography currently within the remit of clinical practice is transient elastography, however, its use is generally outside of the musculoskeletal field. Transient elastography generates a single short impulse of energy, from which the generated shear waves are measured. This is available commercially as a “Fibroscan” for assessing liver fibrosis, but is not currently used in the musculoskeletal system[13].
MUSCULOSKELETAL APPLICATIONS OF ELASTOGRAPHY
Muscles
Skeletal muscles are readily accessible for ultrasound assessment, being superficial in location. Strain elastography has been used to assess many muscle pathologies[14,15], including muscular dystrophy, with a case report showing diseased muscle fibres to be stiffer compared with a normal healthy volunteer[16]. In children with cerebral palsy, elastography has been used to guide botulinum toxin injections into contracted muscles, identified as harder on the elastogram[17]. In myositis, an alteration in the normal muscle elastogram has been shown, with pathological muscles predominantly appearing harder[18]. Within normal muscle, strain elastography has demonstrated greater stiffness in the biceps brachii muscle following exercise compared with pre-exercise elastograms[19]. The postulated cause for this is the physiological response to exercise with increased blood flow and capillary permeability. Between three observers, the authors showed good reliability. A further study reported differences in the stiffness of quadriceps muscle in female subjects with patellofemoral pain syndrome compared with healthy controls[20]. Elastography has also been used in muscular trauma, showing alteration in the normal elastograms with intra-muscular haemorrhage and fibrosis[21]. Both strain and shear wave elastography have been used to assess masseter muscle in healthy volunteers and patients with facial pain[22-25], with good reproducibility between the two techniques. Patients with facial pain and temporomandibular joint dysfunction have been shown to have a greater stiffness in masseter muscle compared with healthy controls. This correlates with the clinical finding of altered hardness of masseter muscle in symptomatic patients with facial pain, thus ultrasound elastography could become a useful adjunct to current techniques in the assessment of these patients[26].
Assessing skeletal muscle with ultrasound elastography, however, has many potential limitations regarding reproducibility. Ensuring a standardised state of contraction/relaxation between assessments is necessary, as is the state of the muscle regarding exercise and rest. Muscles are also subject to anisotropy, so the probe orientation when assessing different muscles must be kept in the same plane (Figure 1). The above factors generate significant challenges with standardisation. In the immediate future elastography alone or in combination with other imaging modalities is unlikely to replace muscle biopsy for the diagnosis of muscular pathologies, namely muscular dystrophy and myositis. The role of elastography in assessing functional muscle disorders is also in its infancy and more research is needed to assess whether it is truly of value or not, given the difficulty with standardisation.
Figure 1.
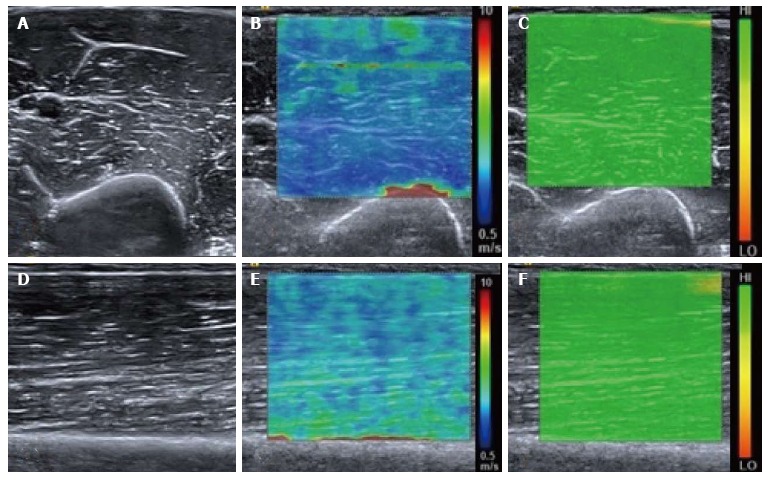
Normal relaxed biceps brachii muscle. B mode transverse image of the mid biceps brachii muscle (A) with the corresponding shear wave elastography image (B) showing the shear wave velocity distribution. The velocity map is coloured such that blue represents the slowest waves and red the fastest, illustrated by the scale. Note the fast (red colour) shear waves at the interface with the hard humeral bone. A range of shear wave velocities are seen at the fascial interfaces within the muscle belly. The upper right image (C) shows the quality map with green colouring representing a high quality elastogram. Corresponding longitudinal B mode image of the mid biceps brachii muscle (D), shear wave velocity image (E) and quality map (F). The velocity distribution is slightly different in the longitudinal plane compared with the transverse plane owing to the greater effect of anisotropy in the transverse plane compared with the longitudinal plane.
Tendons
Elastography allows the possibility of assessing tendons for mucoid degeneration or small interstitial tears potentially before they become apparent on conventional B-mode imaging. When tendons degenerate, the collagen fibres break down and it is proposed that tendons become softer[27], thus a change in their elasticity can be detected with elastography. Conversely, if a tendon repairs with fibrosis, this may be seen as a hardening of the tendon substance, with a stiffer elastographic picture.
The Achilles tendon has received the most attention within elastography studies owing to its ease of accessibility, relatively large size and susceptibility to pathology. It is composed of tightly packed collagen fibres which appear as hypoechoic on B-mode ultrasound with areas of increased reflectivity corresponding to linear interfaces. In symptomatic Achilles tendons there is a disorganisation and breakdown of the collagen fibres, corresponding to mucoid degeneration, and it is proposed that this leads to a softer tendon structure compared with normal[28]. This is shown as alteration in the B-mode architecture, mainly as regions of hypoechogenicity within the tendon substance. The corresponding strain elastography images often show softer regions within the tendon in these areas[29-31]. In patients with surgically repaired Achilles tendons, the regenerative tissue has been shown to be harder and more heterogeneous compared with healthy controls, indicating the reparative tissue to be firmer than normal tendon tissue[30]. Furthermore, patients with ankylosing spondylitis have been shown to have changes in tendon elasticity in strain elastography correlating with B-mode changes, with a difference compared with healthy controls[32].
Shear wave elastography has also been used to study the Achilles tendon. Slower shear wave velocities have been demonstrated in tendinopathic tendons compared with healthy controls[33], also suggesting a softer tendon substance in pathology (Figure 2). As described previously, shear wave elastography measurements are not necessarily comparable between different manufacturers’ equipment, thus potentially limiting the reproducibility. This is evidenced by the large range of quoted normal shear wave values for the Achilles tendon within healthy subjects[34].
Figure 2.
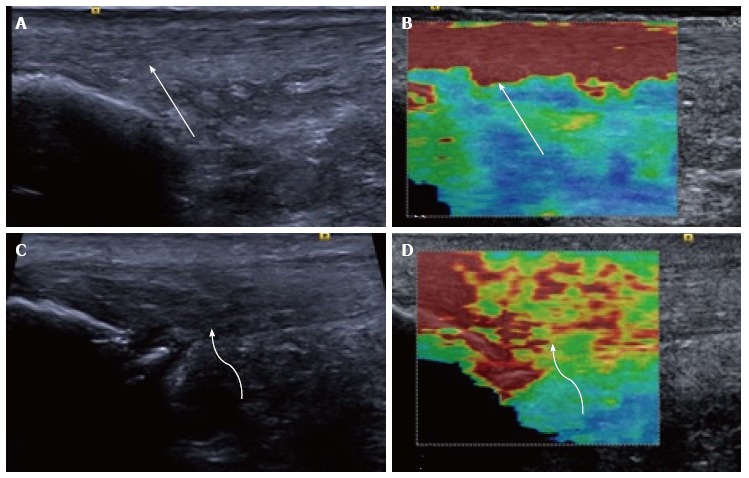
Longitudinal shear wave elastography of the Achilles tendon. In a healthy volunteer the Achilles tendon is seen as smooth and homogeneous (arrow) on the B mode image (A) with a homogeneous elastogram (B, arrow). In a patient with symptomatic Achilles tendinopathy there is an alteration of the B mode echotexture with regions of hypoechogenicity (C, curved arrow) and dystrophic ossification at the calcaneal enthesis. The elastogram (D) is heterogeneous with regions of blue and yellow colouring (curved arrow) corresponding to a slower velocity and tendon softening.
The reproducibility of shear wave elastography has been assessed in tendons with in vivo and in vitro testing. In a study from 2013, repeatability was found to be better in the in vitro setting compared with in vivo, most likely because the in vitro conditions were more amenable to standardisation[35]. Also, the assessed tendon outside the body is static, compared with potential for movement and assessing a different position along the tendon or different probe orientation in human subjects compared with laboratory conditions. A different in vitro study of harvested equine tendons and strain elastography showed poor repeatability between experiments, reflecting the more variable nature of strain elastography compared with shear wave elastography[36].
Away from the Achilles tendon, ultrasound elastography has been used to assess supraspinatus muscle and tendon (Figure 3), with tendinosis predominantly being shown as tendon softening, but also regions of increased stiffness, purported to represent reparative fibrosis (Figures 4 and 5)[37-39]. Strain elastography has also been used around the elbow to assess the common extensor tendon. In a study comparing healthy volunteers with those who were symptomatic, the symptomatic common extensor tendons were shown to be softer compared with healthy volunteers[40].
Figure 3.
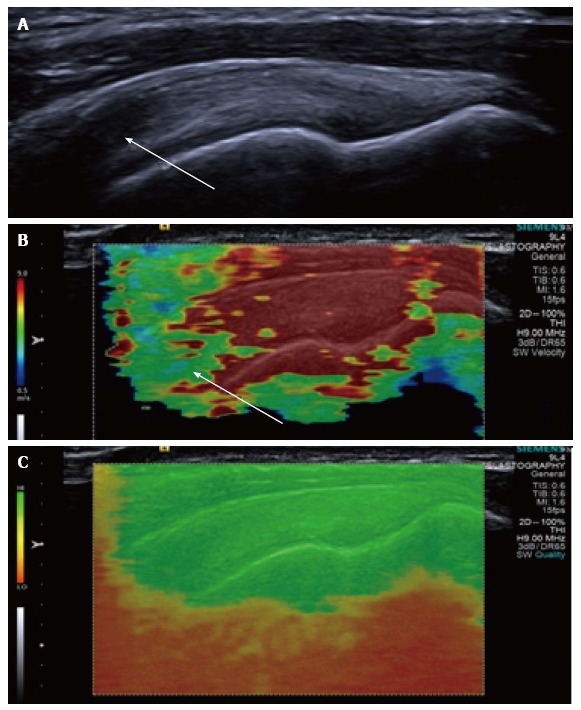
Longitudinal shear wave elastography of a normal supraspinatus tendon. Normal B mode appearances (A) showing anisotropy (arrow) owing to the curved orientation of the tendon. The corresponding elastogram (B) shows heterogeneous stiffness at the region of anisotropy (arrow) and absence of measurements from deep in the humeral head. The quality map (C) shows a high quality elastogram in the tendon substance and poor quality in the bone, as would be expected for such a stiff structure with limited propagation of shear waves.
Figure 4.
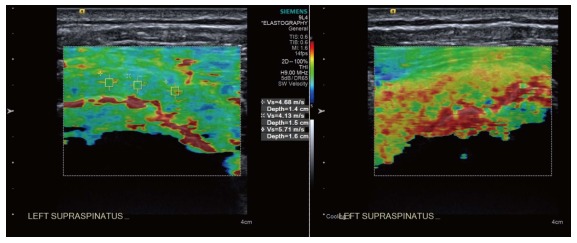
Longitudinal shear wave elastography of a tendinopathic supraspinatus tendon showing a disorganised, heterogeneous pattern, contrasted to the homogeneous appearance of the normal tendon in Figure 3. Note the regions of interest (small boxes) where shear wave velocity measurements have been taken.
Figure 5.
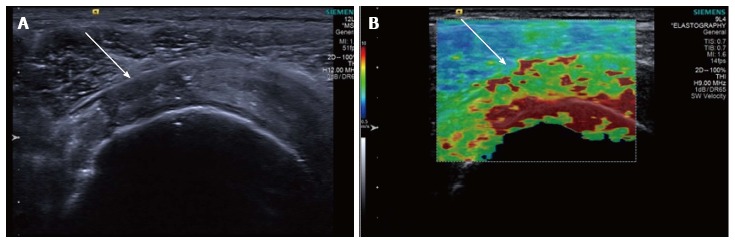
Longitudinal shear wave elastography of a tendinopathic supraspinatus tendon in a different patient to Figure 4. The B mode image (A) shows a partial thickness tear involving the bursal surface fibres (arrow). The corresponding velocity elastogram (B) shows a disorganised pattern with the tear less well delineated (arrow) compared with the B mode image.
Thus, within the tendons the predominant elastographic picture of mucoid degeneration/tendinosis is softening, however, some studies have shown tendinosis as increased tendon stiffness, making a general statement of the elastic properties of tendinosis problematic. The future role of elastography may therefore be to identify areas that have a heterogeneous, disordered elastogram (either softening or hardening) corresponding to tendinosis, rather than the homogeneous elastogram of a normal tendon. This may allow preclinical detection of pathology, potentially of relevance to athletes allowing an alteration in training before injuries occur, or as a screening tool prior to any sporting commitments. Further research is also needed to ascertain if the changes identified on elastography resolve following treatment, or if the tendon becomes asymptomatic. If this were known, then potentially the appearances of a tendon on elastography could be used for prognosis to try and predict the time for tendon healing. Additionally, the elastograms of asymptomatic people of different ages has not been fully characterised and it is not known if the normal ageing process gives rise to different tendon stiffness, or the relevance of alterations in the elastograms in asymptomatic individuals.
Fascia and ligaments
A recent prospective study has shown that the coraco-humeral ligament is stiffer in patients with adhesive capsulitis compared with their unaffected contralateral shoulder. Interestingly, this study also demonstrated variations in the stiffness of the ligament depending on the degree of external rotation of the arm[41]. This is an exciting development, potentially adding additional criteria for diagnosing adhesive capsulitis in addition to the thickness of the coraco-humeral ligament.
Within the plantar fascia, a study using strain elastography found the plantar fascia in symptomatic patients to be thicker and more hypoechoic compared with controls, correlating with a loss of elasticity, or a harder fascia[42].
A further study looking at the stiffness of the A1 pulley in patients with trigger finger showed the pulley to be stiffer in symptomatic patients[43], corresponding with changes in the B-mode appearances too. A further preliminary study has assessed the transverse carpal ligament in the carpal tunnel and the effects of probe pressure on the resultant shear wave velocities. Variations were identified according to the applied probe pressure, indicating that the technique is still somewhat operator dependent[44]. The small size of the finger pulleys and carpal ligaments also significantly limits the use of this technique as the resolution of these small structures can be less than the resolution of the elastograms, particularly with regard to the region of interest for measurement of shear wave velocities within the currently available equipment.
Carpal tunnel
The median nerve at the wrist has been studied with both shear wave elastography and strain elastography[45]. Using strain elastography, the median nerve in patients with carpal tunnel syndrome has been shown to be stiffer compared with controls[46]. These findings are also replicated with shear wave elastography, with a greater shear wave stiffness shown in patients with carpal tunnel syndrome compared with controls[47]. Thus elastography is a useful addition to the B mode appearances and the cross sectional area of the median nerve in diagnosing carpal tunnel syndrome, with potential to replace nerve conduction studies as the diagnostic gold standard.
Joints
Very little information is available on elastography of the joints, in particular, the synovium. To date, there are no published clinical trials assessing elastography in the joints. Case reports of strain elastography in a small number of patients suggests that inflammatory synovitis may appear as firm, compared with infective synovitis which may appear as firm to soft. Thus, there is overlap in the elastographic appearances between infective and inflammatory synovitis and the technique is unable to replace the gold standard of tissue diagnosis for either infective or inflammatory synovitis[48,49].
There is no data published on the elastographic appearances of the synovium in normal subjects vs those with an inflammatory arthropathy (Figure 6). This is an area requiring further research, to determine if elastography can be as useful a tool as Doppler in the assessment of suspected inflammatory synovitis and potential response to disease modifying anti-rheumatic therapies.
Figure 6.
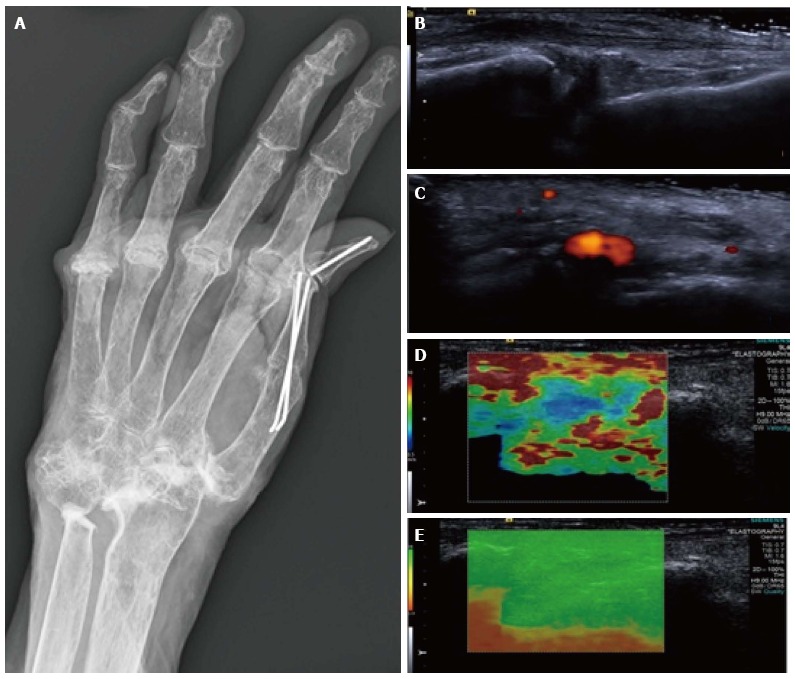
Synovitis at the carpus. Radiograph (A) in a patient with long-standing rheumatoid arthritis and worsening wrist pain. The longitudinal B mode image (B) and colour Doppler image (C) show hypoechogenicity of the synovium with increased Doppler flow indicating an active synovitis. The corresponding longitudinal shear wave velocity elastogram (D) shows relatively uniformly soft synovium (blue colour) compared with the adjacent tissues which are more heterogeneous in their stiffness. The significance of this has not yet been adequately investigated. The elastogram is of good quality (E).
Tumours
Elastography in tumours has been used extensively outside the musculoskeletal system, predominantly within breast, thyroid and prostate lesions. Within the breast, elastography is proving to be useful to help differentiate between benign and malignant lesions (including threshold values for shear wave velocities in malignant lesions) however, within the thyroid there is significant overlap in the elastographic characteristics between the two.
In musculoskeletal soft tissue tumours, elastography has been used to prospectively assess lesions to attempt to differentiate between benign and malignant pathologies. Malignant lesions have been shown to be stiffer on strain elastography compared with benign lesions on a semi-quantitative scale, similar to lesions outside of the musculoskeletal system[50]. However, a recently published study by the group in Leeds assessing the role of shear wave elastography in musculoskeletal tumours has been unable to replicate these early findings, with the authors concluding that there is currently no additional role for shear wave elastography in soft tissue tumours compared with B mode imaging[51]. The authors found no statistically significant association between shear wave velocity and malignancy. According to the strain elastography study, one may have expected malignant tumours to be stiffer than benign tumours and thus have a tendency for higher shear wave velocities. Possibly the discordant results are accounted for by the tumour case mix, different elastography technique or study design. Soft tissue tumours are very heterogeneous on B mode imaging (Figure 7) compared with breast lesions and it is probable that further studies will replicate the findings of the Leeds group, showing a large overlap in the shear wave velocity measurements between benign and malignant soft tissue lesions. In the immediate future imaging characteristics alone, even with the addition of elastography, are unlikely to replace biopsy for the diagnosis of malignant vs benign lesions. Similar to tendons, the role of elastography in soft tissue tumours is likely to be in identifying a heterogeneous, disorganised internal substance suggesting an aggressive nature or de-differentiation, vs a smooth homogeneous pattern of a non-aggressive lesion. This could be used to guide biopsy by potentially identifying aggressive regions within a lesion, or to identify malignant degeneration in a previously diagnosed benign lesion.
Figure 7.
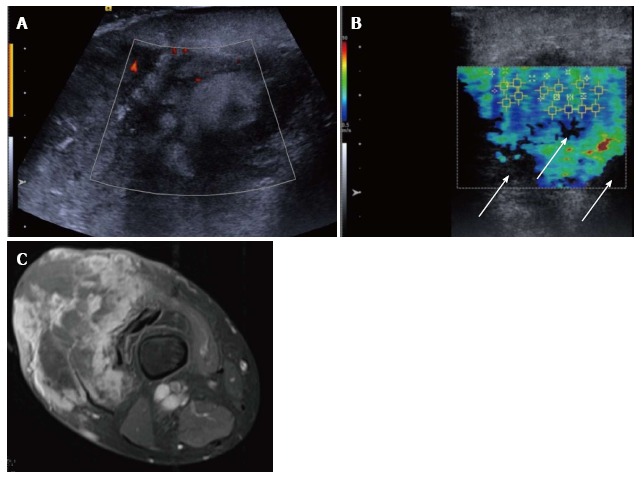
Pleomorphic sarcoma of the distal thigh. Transverse B mode image with colour Doppler (A) shows a disorganised tumour with wide variation in echotexture and limited Doppler flow. The shear wave velocity elastogram (B) also shows a wide variation in stiffness with some regions of absent measurements, seen as the black areas on the image (arrow), possibly owing to the very dense/stiff nature of the tumour. The axial proton density fat suppressed magnetic resonance image (C) shows a large tumour in the anterior compartment of the thigh with very varied signal.
Another potential use of elastography in tumours may be to define the boundaries of a lesion, compared with normal tissue. In regions with poor B-mode contrast it can be hard to identify the margin of a lesion, however, the elastogram may show a sharp demarcation if the lesion has different elastic properties compared with the adjacent normal tissue (Figure 8). This is of value within breast[52] and prostate lesions and may prove to be the most useful application of elastography in soft tissue tumours.
Figure 8.
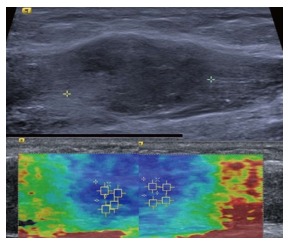
Intramuscular nodular fasciitis, longitudinal images. The B mode image (upper image) shows that the margins of the lesion are poorly defined and relatively hard to discern. The shear wave velocity elastograms (lower images) show a clear difference in stiffness between the lesion and adjacent normal muscle fibres, as shown by the blue (slow) colour map of the lesion compared with the green and red (faster) colour map of the adjacent muscle fibres.
ELASTOGRAPHY OUTSIDE THE MUSCULOSKELETAL SYSTEM
Elastography has been extensively used in structures outside the musculoskeletal system, namely the liver, breast, thyroid and prostate[53-60]. Within the liver, it has now become standard in the assessment of fibrosis, replacing biopsy in many instances. In the breast, thyroid and prostate, elastography has become part of the non-invasive assessment of a lesion, being used in addition to the B mode and Doppler characteristics[61-63]. Generally, aggressive cancers with more malignant features have been shown to have a greater stiffness as measured by shear wave elastography compared with less aggressive tumours[64]. However, despite the recent advances in tumour characterisation with elastography and other imaging parameters, the gold standard of biopsy has not yet been replaced by imaging alone for the majority of lesions and it this is unlikely to be the case in the near future.
FUTURE DIRECTIONS
Technical
As with all technological advances, time will bring refinements in the current technology, perhaps allowing shear wave measurements at greater depths, or within very superficial structures such as skin.
Elastography adds another facet to the available ultrasound capabilities and may potentially be fused with other imaging modalities, namely CT and MRI. This could then create a navigable data set to aid with surgical planning or to guide tumour resection, particularly with reference to tumour margins.
Clinical applications
Within the musculoskeletal system ultrasound elastography has the potential to become as valuable a tool as within the liver, breast and thyroid. It may allow identification of preclinical tendinosis and thus modification of activity or prophylactic treatment prior to symptoms developing. It could be used to predict time to recovery following treatment and help guide the timing of return to activity, once the tendon elastogram returns to normal.
Elastography could ultimately obviate the need for biopsy in soft tissue tumours enabling differentiation between benign and malignant lesions, although as described above, further research is needed. Following tumour resection it may help distinguish post-surgical scarring from recurrent tumour, with the scarring being harder and more homogeneous than tumour.
Elastography could possibly be used to assess the state of cartilage in areas accessible to the ultrasound probe, such as the shoulder and talar dome. Cartilage which is degenerate may display different elastic properties, similar to DGEMRIC and T2 rho magnetic resonance imaging (MRI), thus providing a cheaper alternative to MRI. Within the synovium, if the synovitis of inflammatory arthropathies displays reliable alterations in elasticity compared with normal synovium, it may form part of the routine assessment and synovitis grading, similar to power Doppler.
Within current parameters, elastography data from bone is limited as it is virtually incompressible. If the technology could be refined to detect even the smallest deformation in bone or fracture callus, it could potentially be used to assess when a fracture callus has matured enough to withstand weight-bearing, or to similarly assess the fusion mass following spinal surgery.
CONCLUSION
Ultrasound elastography is an exciting new development within clinical practice. Strain elastography has created maps of tissue stiffness, in addition to the B mode and Doppler characteristics. The arrival of shear wave elastography has brought a quantitative assessment to elastography and introduced a technique which is potentially less operator dependent compared with strain elastography. The use of ultrasound elastography in the musculoskeletal system is likely to become as widespread as within the liver, breast and thyroid now that more clinical departments have access to this technology. With greater clinical usage and research, its applications will be verified, validated and widened. However in the immediate future the role of ultrasound elastography is likely to be complementary to conventional imaging techniques in providing an additional tool, rather than replacing anything that is current standard practice.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: June 29, 2016
First decision: August 5, 2016
Article in press: October 28, 2016
P- Reviewer: Razek AAKA, Schoenhagen P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Ophir J, Alam SK, Garra BS, Kallel F, Konofagou EE, Krouskop T, Merritt CR, Righetti R, Souchon R, Srinivasan S, et al. Elastography: Imaging the elastic properties of soft tissues with ultrasound. J Med Ultrason (2001) 2002;29:155. doi: 10.1007/BF02480847. [DOI] [PubMed] [Google Scholar]
- 2.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487–495. doi: 10.1016/j.diii.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LS, Porter BC, Rubens DJ, Parker KJ. Three-dimensional sonoelastography: principles and practices. Phys Med Biol. 2000;45:1477–1494. doi: 10.1088/0031-9155/45/6/306. [DOI] [PubMed] [Google Scholar]
- 4.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85:1435–1445. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol. 2010;14:323–333. doi: 10.1055/s-0030-1254521. [DOI] [PubMed] [Google Scholar]
- 6.Benson J. Understanding ARFI and New Elastography Quantification Technologies. Available from: http://usa.healthcare.siemens.com/ultrasound/understanding-arfi.
- 7.Elkateb Hachemi M, Callé S, Remenieras JP. Transient displacement induced in shear wave elastography: comparison between analytical results and ultrasound measurements. Ultrasonics. 2006;44 Suppl 1:e221–e225. doi: 10.1016/j.ultras.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Kot BC, Zhang ZJ, Lee AW, Leung VY, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One. 2012;7:e44348. doi: 10.1371/journal.pone.0044348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa) Ultrasonography. 2014;33:34–39. doi: 10.14366/usg.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol. 2014;59:505–523. doi: 10.1088/0031-9155/59/3/505. [DOI] [PubMed] [Google Scholar]
- 11.Woo H, Lee JY, Yoon JH, Kim W, Cho B, Choi BI. Comparison of the Reliability of Acoustic Radiation Force Impulse Imaging and Supersonic Shear Imaging in Measurement of Liver Stiffness. Radiology. 2015;277:881–886. doi: 10.1148/radiol.2015141975. [DOI] [PubMed] [Google Scholar]
- 12.Arda K, Ciledag N, Aribas BK, Aktas E, Köse K. Quantitative assessment of the elasticity values of liver with shear wave ultrasonographic elastography. Indian J Med Res. 2013;137:911–915. [PMC free article] [PubMed] [Google Scholar]
- 13.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Ultrasound Q. 2016;32:94–107. doi: 10.1097/RUQ.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 14.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshitake Y, Takai Y, Kanehisa H, Shinohara M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve. 2014;50:103–113. doi: 10.1002/mus.24104. [DOI] [PubMed] [Google Scholar]
- 16.Drakonaki EE, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39:391–396. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 17.Vasilescu D, Vasilescu D, Dudea S, Botar-Jid C, Sfrângeu S, Cosma D. Sonoelastography contribution in cerebral palsy spasticity treatment assessment, preliminary report: a systematic review of the literature apropos of seven patients. Med Ultrason. 2010;12:306–310. [PubMed] [Google Scholar]
- 18.Botar-Jid C, Damian L, Dudea SM, Vasilescu D, Rednic S, Badea R. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason. 2010;12:120–126. [PubMed] [Google Scholar]
- 19.Yanagisawa O, Niitsu M, Kurihara T, Fukubayashi T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: a feasibility study. Clin Radiol. 2011;66:815–819. doi: 10.1016/j.crad.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Botanlioglu H, Kantarci F, Kaynak G, Unal Y, Ertan S, Aydingoz O, Erginer R, Unlu MC, Mihmanli I, Babacan M. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skeletal Radiol. 2013;42:659–666. doi: 10.1007/s00256-012-1520-4. [DOI] [PubMed] [Google Scholar]
- 21.Botar Jid C, Vasilescu D, Damian L, Dumitriu D, Ciurea A, Dudea SM. Musculoskeletal sonoelastography. Pictorial essay. Med Ultrason. 2012;14:239–245. [PubMed] [Google Scholar]
- 22.Nakayama M, Ariji Y, Nishiyama W, Ariji E. Evaluation of the masseter muscle elasticity with the use of acoustic coupling agents as references in strain sonoelastography. Dentomaxillofac Radiol. 2015;44:20140258. doi: 10.1259/dmfr.20140258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariji Y, Nakayama M, Nishiyama W, Nozawa M, Ariji E. Shear-wave sonoelastography for assessing masseter muscle hardness in comparison with strain sonoelastography: study with phantoms and healthy volunteers. Dentomaxillofac Radiol. 2016;45:20150251. doi: 10.1259/dmfr.20150251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariji Y, Gotoh A, Hiraiwa Y, Kise Y, Nakayama M, Nishiyama W, Sakuma S, Kurita K, Ariji E. Sonographic elastography for evaluation of masseter muscle hardness. Oral Radiol. 2013;29:64–69. [Google Scholar]
- 25.Ariji Y, Nakayama M, Taguchi A, Gotoh A, Kise Y, Katsumata A, Kurita K, Ariji E. Intramuscular changes of soft and hard areas after low-level static contraction of the masseter muscle and the correlations with muscle hardness and increase in water content: evaluations with sonographic elastography and magnetic resonance imaging. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:354–361. doi: 10.1016/j.oooo.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Kashima K, Igawa K, Maeda S, Sakoda S. Analysis of muscle hardness in patients with masticatory myofascial pain. J Oral Maxillofac Surg. 2006;64:175–179. doi: 10.1016/j.joms.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 28.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Zordo T, Chhem R, Smekal V, Feuchtner G, Reindl M, Fink C, Faschingbauer R, Jaschke W, Klauser AS. Real-time sonoelastography: findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31:394–400. doi: 10.1055/s-0028-1109809. [DOI] [PubMed] [Google Scholar]
- 30.Tan S, Kudaş S, Özcan AS, İpek A, Karaoğlanoğlu M, Arslan H, Bozkurt M. Real-time sonoelastography of the Achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skeletal Radiol. 2012;41:1067–1072. doi: 10.1007/s00256-011-1339-4. [DOI] [PubMed] [Google Scholar]
- 31.Ooi CC, Schneider ME, Malliaras P, Counsel P, Connell DA. Prevalence of morphological and mechanical stiffness alterations of mid Achilles tendons in asymptomatic marathon runners before and after a competition. Skeletal Radiol. 2015;44:1119–1127. doi: 10.1007/s00256-015-2132-6. [DOI] [PubMed] [Google Scholar]
- 32.Turan A, Tufan A, Mercan R, Teber MA, Tezcan ME, Bitik B, Goker B, Haznedaroğlu S. Real-time sonoelastography of Achilles tendon in patients with ankylosing spondylitis. Skeletal Radiol. 2013;42:1113–1118. doi: 10.1007/s00256-013-1637-0. [DOI] [PubMed] [Google Scholar]
- 33.Aubry S, Nueffer JP, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology. 2015;274:821–829. doi: 10.1148/radiol.14140434. [DOI] [PubMed] [Google Scholar]
- 34.Chen XM, Cui LG, He P, Shen WW, Qian YJ, Wang JR. Shear wave elastographic characterization of normal and torn achilles tendons: a pilot study. J Ultrasound Med. 2013;32:449–455. doi: 10.7863/jum.2013.32.3.449. [DOI] [PubMed] [Google Scholar]
- 35.Peltz CD, Haladik JA, Divine G, Siegal D, van Holsbeeck M, Bey MJ. ShearWave elastography: repeatability for measurement of tendon stiffness. Skeletal Radiol. 2013;42:1151–1156. doi: 10.1007/s00256-013-1629-0. [DOI] [PubMed] [Google Scholar]
- 36.Buck AR, Verstraete N, Li Y, Schweizer A, Snedeker JG, Buck FM. Detection of small tendon lesions by sonoelastographic visualization of strain profile differences: initial experiences. Skeletal Radiol. 2012;41:1073–1079. doi: 10.1007/s00256-011-1349-2. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zhan W, Zhou M, Zhang X. Ultrasound elastography of the supraspinatus tendon guided by US-MRI virtual navigation. Technol Health Care. 2015;23 Suppl 2:S263–S268. doi: 10.3233/THC-150961. [DOI] [PubMed] [Google Scholar]
- 38.Muraki T, Ishikawa H, Morise S, Yamamoto N, Sano H, Itoi E, Izumi S. Ultrasound elastography-based assessment of the elasticity of the supraspinatus muscle and tendon during muscle contraction. J Shoulder Elbow Surg. 2015;24:120–126. doi: 10.1016/j.jse.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Rosskopf AB, Ehrmann C, Buck FM, Gerber C, Flück M, Pfirrmann CW. Quantitative Shear-Wave US Elastography of the Supraspinatus Muscle: Reliability of the Method and Relation to Tendon Integrity and Muscle Quality. Radiology. 2016;278:465–474. doi: 10.1148/radiol.2015150908. [DOI] [PubMed] [Google Scholar]
- 40.De Zordo T, Lill SR, Fink C, Feuchtner GM, Jaschke W, Bellmann-Weiler R, Klauser AS. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193:180–185. doi: 10.2214/AJR.08.2020. [DOI] [PubMed] [Google Scholar]
- 41.Wu CH, Chen WS, Wang TG. Elasticity of the Coracohumeral Ligament in Patients with Adhesive Capsulitis of the Shoulder. Radiology. 2016;278:458–464. doi: 10.1148/radiol.2015150888. [DOI] [PubMed] [Google Scholar]
- 42.Sconfienza LM, Silvestri E, Orlandi D, Fabbro E, Ferrero G, Martini C, Sardanelli F, Cimmino MA. Real-time sonoelastography of the plantar fascia: comparison between patients with plantar fasciitis and healthy control subjects. Radiology. 2013;267:195–200. doi: 10.1148/radiol.12120969. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto H, Miura T, Isayama H, Masuzaki R, Koike K, Ohe T. Stiffness of the first annular pulley in normal and trigger fingers. J Hand Surg Am. 2011;36:1486–1491. doi: 10.1016/j.jhsa.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 44.Shen ZL, Vince DG, Li ZM. In vivo study of transverse carpal ligament stiffness using acoustic radiation force impulse (ARFI) imaging. PLoS One. 2013;8:e68569. doi: 10.1371/journal.pone.0068569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonagh C, Alexander M, Kane D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: a new paradigm. Rheumatology (Oxford) 2015;54:9–19. doi: 10.1093/rheumatology/keu275. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto H, Halpern EJ, Kastlunger M, Gabl M, Arora R, Bellmann-Weiler R, Feuchtner GM, Jaschke WR, Klauser AS. Carpal tunnel syndrome: diagnosis by means of median nerve elasticity--improved diagnostic accuracy of US with sonoelastography. Radiology. 2014;270:481–486. doi: 10.1148/radiol.13122901. [DOI] [PubMed] [Google Scholar]
- 47.Kantarci F, Ustabasioglu FE, Delil S, Olgun DC, Korkmazer B, Dikici AS, Tutar O, Nalbantoglu M, Uzun N, Mihmanli I. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol. 2014;24:434–440. doi: 10.1007/s00330-013-3023-7. [DOI] [PubMed] [Google Scholar]
- 48.Lalitha P, Reddy B. Synovial Sonoelastography: Utility in differentiating between inflammatory and infective synovitis- a comparative study with magnetic resonance imaging. Eur Soc Radiol. 2011:C–0470. [Google Scholar]
- 49.Lalitha P, Reddy MCh, Reddy KJ. Musculoskeletal applications of elastography: a pictorial essay of our initial experience. Korean J Radiol. 2011;12:365–375. doi: 10.3348/kjr.2011.12.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magarelli N, Carducci C, Bucalo C, Filograna L, Rapisarda S, De Waure C, Dell’Atti C, Maccauro G, Leone A, Bonomo L. Sonoelastography for qualitative and quantitative evaluation of superficial soft tissue lesions: a feasibility study. Eur Radiol. 2014;24:566–573. doi: 10.1007/s00330-013-3069-6. [DOI] [PubMed] [Google Scholar]
- 51.Pass B, Jafari M, Rowbotham E, Hensor EMA, Gupta H, Robinson P. Do quantitative and qualitative shear wave elastography have a role in evaluating musculoskeletal soft tissue masses? Eur Radiol. 2016 doi: 10.1007/s00330-016-4427-y. Jun 8; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullen R, Thompson JM, Moussa O, Vinnicombe S, Evans A. Shear-wave elastography contributes to accurate tumour size estimation when assessing small breast cancers. Clin Radiol. 2014;69:1259–1263. doi: 10.1016/j.crad.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Madhok R, Tapasvi C, Prasad U, Gupta AK, Aggarwal A. Acoustic radiation force impulse imaging of the liver: measurement of the normal mean values of the shearing wave velocity in a healthy liver. J Clin Diagn Res. 2013;7:39–42. doi: 10.7860/JCDR/2012/5070.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F, et al. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240–247. doi: 10.1111/j.1365-2893.2012.01646.x. [DOI] [PubMed] [Google Scholar]
- 55.Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol. 2013;43:539–544. doi: 10.1007/s00247-012-2553-5. [DOI] [PubMed] [Google Scholar]
- 56.D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Gallotti A, Pozzi Mucelli R. Acoustic radiation force impulse of the liver. World J Gastroenterol. 2013;19:4841–4849. doi: 10.3748/wjg.v19.i30.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzmán-Aroca F, Frutos-Bernal MD, Bas A, Luján-Mompeán JA, Reus M, Berná-Serna Jde D, Parrilla P. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol. 2012;22:2525–2532. doi: 10.1007/s00330-012-2505-3. [DOI] [PubMed] [Google Scholar]
- 58.Ying L, Lin X, Xie ZL, Tang FY, Hu YP, Shi KQ. Clinical utility of acoustic radiation force impulse imaging for identification of malignant liver lesions: a meta-analysis. Eur Radiol. 2012;22:2798–2805. doi: 10.1007/s00330-012-2540-0. [DOI] [PubMed] [Google Scholar]
- 59.Şirli R, Sporea I, Bota S, Raţiu I. Liver elastography for the diagnosis of portal hypertension in patients with liver cirrhosis. Med Ultrason. 2012;14:225–230. [PubMed] [Google Scholar]
- 60.Ginat DT, Destounis SV, Barr RG, Castaneda B, Strang JG, Rubens DJ. US elastography of breast and prostate lesions. Radiographics. 2009;29:2007–2016. doi: 10.1148/rg.297095058. [DOI] [PubMed] [Google Scholar]
- 61.Tozaki M, Isobe S, Sakamoto M. Combination of elastography and tissue quantification using the acoustic radiation force impulse (ARFI) technology for differential diagnosis of breast masses. Jpn J Radiol. 2012;30:659–670. doi: 10.1007/s11604-012-0106-3. [DOI] [PubMed] [Google Scholar]
- 62.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Baker L, Jordan L, Rauchhaus P, Thompson A. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology. 2012;263:673–677. doi: 10.1148/radiol.12111317. [DOI] [PubMed] [Google Scholar]
- 63.Correas JM, Tissier AM, Khairoune A, Vassiliu V, Méjean A, Hélénon O, Memo R, Barr RG. Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology. 2015;275:280–289. doi: 10.1148/radiol.14140567. [DOI] [PubMed] [Google Scholar]
- 64.Bojunga J, Dauth N, Berner C, Meyer G, Holzer K, Voelkl L, Herrmann E, Schroeter H, Zeuzem S, Friedrich-Rust M. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One. 2012;7:e42735. doi: 10.1371/journal.pone.0042735. [DOI] [PMC free article] [PubMed] [Google Scholar]


