Abstract
Saponins are triterpene glycoside natural products that exhibit many different biological properties, including activation and modulation of the immune system, and have therefore attracted significant interest as immunological adjuvants for use in vaccines. QS-21 is the most widely used and promising saponin adjuvant but suffers from several liabilities, such as scarcity, dose-limiting toxicity, and hydrolytic instability. Chemical synthesis has emerged as a powerful approach to obtain homogeneous, pure samples of QS-21 and to improve its properties and therapeutic profile by providing access to optimized, synthetic saponin variants. Herein, we describe a general method for the semisynthesis of these molecules from QS-21, with detailed synthetic protocols for two saponin variants developed in our recent work.
Keywords: Vaccine, Immunoadjuvant, QS-21, Saponin, Natural product, Semisynthesis, Carbohydrate, Glycosylation
1 Introduction
Saponins are a class of plant-derived, natural products consisting of a triterpenoid or steroidal core glycosylated with a variety of sugar units and having important biological and pharmacological properties. Saponin extracts from the bark of the South American tree Quillaja saponaria (QS) have long been known for their immunoadjuvant activities. A purified QS-21 fraction from this extract [1] has been widely used as an adjuvant to potentiate the immune response to classical whole-organism and modern subunit vaccines. Despite the unique potency and promise of QS-21 in numerous vaccine clinical trials, its inherent limitations in terms of scarcity and heterogeneity, dose-limiting toxicity, and chemical instability [2] have hindered its further clinical advancement, with the notable exceptions of the recent malaria (Mosquirix) and shingles vaccines developed by GSK [3, 4]. Structural modification of the natural product at the molecular level via de novo chemical synthesis has become a powerful approach to address these liabilities.
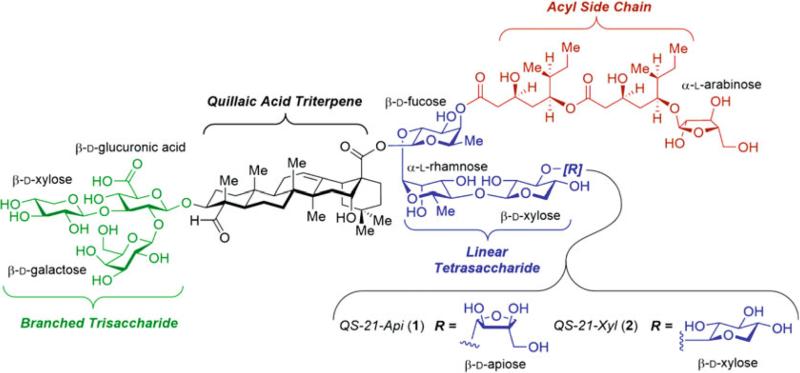
QS-21 (Fig. 1) comprises four structural domains, with a central quillaic acid triterpene core flanked by a branched trisaccharide, a bridging linear tetrasaccharide, and a glycosylated acyl side chain. The natural product is not a single compound but a ≈2:1 mixture of apiose and xylose isomers (QS-21-Api, 1 and QS-21-Xyl, 2) at the terminal sugar in the linear tetrasaccharide domain. The total syntheses of each of these QS-21 isomers provided a robust method to produce this potent adjuvant in high purity and homogeneous composition [5–7]. These efforts involved the synthesis of all four structural domains of QS-21 in protected form, followed by late-stage coupling, global deprotection, and HPLC purification to provide synthetic QS-21-Api [5, 6] and QS-21-Xyl [7] in a highly modular fashion. To streamline the rather lengthy, multistep preparation of these molecules, a novel semisynthetic route was then developed [8]. In this route, the branched trisaccharide–triterpene conjugate (prosapogenin), which is common to both QS-21 isomers, was obtained via basic hydrolysis and chromatographic purification of commercially available Quil-A, a semipurified, saponin-rich extract from Q. saponaria. Installation of the bridging linear tetrasaccharide and acyl chain domains then provided access to a variety of QS-21 analogues.
Fig. 1.
Chemical structure of the QS-21 saponin adjuvant and its four structural domains
The technology gained from the chemical synthesis of QS-21 and the establishment of the new semisynthetic route has enabled the application of this saponin chemistry to design and synthesize novel, synthetically accessible QS-21 variants that exhibit increased stability and potency, and attenuated in vivo toxicity [9–14]. Our studies have revealed that significant structural modifications of the bridging fucose residue within the linear tetrasaccharide and of the acyl chain of QS-21 cause little or no impairment of adjuvant activity [9, 10, 12]. Additionally, systematic structure–function studies of the carbohydrate regions have indicated that the terminal sugar within the linear tetrasaccharide as well as the entire branched trisaccharide domain are not required for adjuvant activity [10, 11]. This led us to identify highly simplified acyl chain variants and a truncated linear trisaccharide congener that were equipotent to QS-21 in a preclinical vaccination model. More recently, structure–activity investigations around the central glycosidic ester linkage revealed that relatively conservative modifications result in significant differences in adjuvant activity that correlate with local and global conformational changes observed in molecular dynamics studies [14].
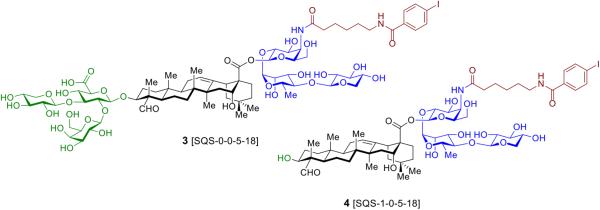
In this chapter, we present a general approach to the semisynthesis of saponin variants based on the QS-21 parent architecture. Syntheses of two saponin adjuvants, 3 (SQS-0-0-5-18) and 4 (SQS-1-0-5-18) (Fig. 2), which include and lack the branched trisaccharide domain, respectively, are provided as examples. We describe protocols for the controlled hydrolysis and purification of Quillaja extracts to isolate sufficient quantities of the prosapogenin and triterpene substructures of QS-21 and their selective protection. We also detail synthetic routes to the requisite structural domains (prosapogenin and triterpene substructures, as well as simplified acyl chain and truncated linear oligosaccharide domains) and their late-stage, convergent assembly to protected saponin scaffolds, followed by global deprotection.
Fig. 2.
Chemical structures of semisynthetic saponin adjuvants based on QS-21
2 Materials
All chemicals are obtained from commercial vendors (Aldrich Chemical, Acros Organics, or Fisher Scientific) and used without further purification unless otherwise noted.
Lyophilized QS saponin Quil A, obtained from Brenntag Biosector (Frederikssund, Denmark) via distribution by Accurate Chemical and Scientific Corporation (Westbury, NY).
Dichloromethane (DCM), tetrahydrofuran (THF), acetonitrile (MeCN), toluene, and benzene, purified by passage through two packed columns of neutral alumina under an argon (Ar) atmosphere using a solvent drying system.
Methanol (MeOH), distilled from magnesium turnings under nitrogen (N2) atmosphere at 760 Torr.
N,N-Dimethylformamide (DMF), dried over freshly activated 4 Å molecular sieves (MS) or as purchased from Acros Organics (N,N-dimethylformamide 99.8 %, Extra Dry over Molecular Sieve, AcroSeal™).
Triethylamine (Et3N), pyridine, and boron trifluoride diethyl etherate (BF3·OEt2), distilled from calcium hydride (CaH2) under N2 atmosphere at 760 Torr.
Trifluoromethanesulfonic anhydride (Tf2O), distilled from phosphorus pentoxide (P2O5) under N2 atmosphere at 760 Torr.
Acetic acid (AcOH).
Acetone.
Acetyl chloride (AcCl).
Activated charcoal.
Allyl alcohol.
Allyl bromide.
Aluminum foil.
Benzoyl chloride (BzCl).
Benzyl bromide (BnBr).
Benzyl chloroformate (CbzCl).
Brine (saturated solution of sodium chloride (NaCl) in distilled water).
tert-Butanol (tBuOH).
6-[(tert-Butoxycarbonyl)amino]hexanoic acid (6-(Boc-amino) hexanoic acid).
Celite.
Chloroform (CHCl3).
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU).
Diethyl ether (Et2O).
Diethylamine (Et2NH).
2,2-Dimethoxypropane (DMP).
4-Dimethylaminopyridine (DMAP).
Diphenyldiselenide (PhSeSePh).
Ethanol (EtOH).
Ethyl acetate (EtOAc).
Ethyl chloroformate (EtOCOCl).
d-Glucal.
Hydrochloric acid, concentrated (HCl, 37 %, 12.1 N).
HCl (1 N aqueous).
Hexanes.
Hypophosphorous acid (H3PO2), 50 % solution in water.
Imidazole.
4-Iodobenzoic acid N-hydroxysuccinimide ester.
2,6-Lutidine.
Magnesium sulfate (MgSO4).
Methanesulfonyl chloride (MsCl).
Methanol (MeOH).
Methanol-d4 (deuterated methanol).
N-Methylmorpholine N-oxide (NMO).
Osmium tetroxide (OsO4).
Palladium (II) acetate (Pd(OAc)2).
Palladium on carbon (Pd/C), 10 % dry basis, wet Degussa type E101 NE/W.
Phenylselenol (PhSeH).
Phenyl sulfoxide (Ph2SO).
Potassium bicarbonate (KHCO3).
Potassium hydroxide (KOH).
Pyrrolidine.
l-Rhamnose monohydrate.
Sodium azide (NaN3).
Sodium bicarbonate (NaHCO3).
NaHCO3, saturated solution in distilled water.
Sodium hydride (NaH), 60 % dispersion in mineral oil.
Sodium hydroxide (NaOH).
Sodium sulfate (Na2SO4).
Sodium sulfite (Na2SO3).
Tetrabutylammonium chloride (Bu4NCl).
Tetrabutylammonium fluoride (TBAF), solution in THF (1 M).
Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4).
p-Toluenesulfonic acid monohydrate (pTsOH).
Trichloroacetonitrile (Cl3CCN).
Triethylsilyltrifluoromethanesfulonate (TESOTf).
Trifluoroacetic acid (TFA).
Triisopropylsilyl chloride (TIPSCl).
Triphenylphosphine (PPh3).
Water, distilled.
d-Xylose.
3 Methods
3.1 General Experimental Methods
Reactions are performed in a flame-dried, modified Schlenk flask (Kjeldahl shape), equipped with a Teflon-coated, magnetic stir bar, and fitted with a glass stopper under positive pressure of Ar, unless otherwise noted.
Air- and moisture-sensitive liquids are transferred via glass syringe or stainless-steel cannula, which are oven-dried and cooled under Ar prior to use.
Gas-tight syringes are dried and stored in a desiccator and purged with Ar prior to use.
Carbohydrate and sulfoxide reagents are dried via azeotropic removal of water with toluene (~2 mL/0.10 mmol scale) under high vacuum. This process is repeated three times and the residue is further dried under vacuum.
Molecular sieves (MS) are activated at 350 °C, then crushed and flame-dried under vacuum immediately prior to use.
2,4,6-Tri-tert-butylpyridine (TBP) used for dehydrative glycosylation reactions is stored and weighed out in a glovebox, then added from a vial under Ar.
Cooling baths are generated as follows: 0 °C, wet ice/water; −10 °C, wet ice/acetone; −42 °C, dry ice/EtOH:water (1:1) or dry ice/MeCN; −60 °C, dry ice/CHCl3; −78 °C, dry ice/acetone.
Organic solutions are concentrated by rotary evaporation below 35 °C.
Analytical thin-layer chromatography (TLC) is performed using glass plates precoated to a depth of 0.25 mm with 230–400 mesh silica gel (E. Merck) impregnated with a fluorescent indicator (254 nm). Compounds are visualized using UV light (254 nm) or by dipping the plates in a ceric ammonium molybdate (CAM) solution followed by heating.
Flash column chromatography is performed using 230–400 mesh silica gel (E. Merck) under positive pressure of nitrogen.
Automated silica gel column chromatography is performed on an Isco Combiflash Rf with Isco columns (Teledyne Isco, Lincoln, Nebraska). Gradient elutions are performed with a linear gradient of the indicated solvents.
Reverse-phase high performance liquid chromatography (RP-HPLC) purification and analyses are carried out on a Waters 2545 binary gradient HPLC system equipped with a Waters 2996 photodiode array detector (Waters Corporation, Milford, Massachusetts). Absorbances are monitored at wavelengths of 210–600 nm. RP-HPLC purifications are performed on an XBridge Prep BEH300 C18 column (5 μm, 10 × 250 mm) using a linear gradient of acetonitrile/water at a flow rate of 5 mL/min.
3.2 Isolation and Selective Protection of Branched Trisaccharide–Triterpene Prosapogenin
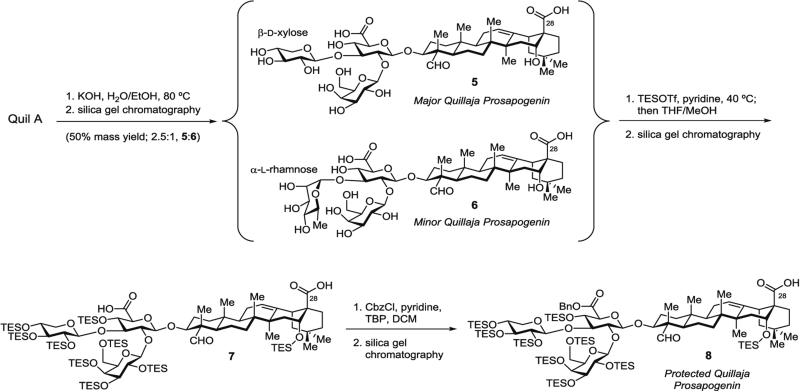
3.2.1 Isolation of Branched Trisaccharide–Triterpene Prosapogenins 5 and 6 (Fig. 3) from Quil A
Fig. 3.
Isolation and selective protection of branched trisaccharide–triterpene prosapogenin
In a 250-mL round-bottomed flask equipped with a reflux condenser, Quil A (1.15 g) and potassium hydroxide (0.97 g, 17 mmol) are suspended in EtOH/water (1:1) (50 mL), then the mixture is heated to 80 °C for 7 h.
The reaction is cooled to 0 °C, neutralized with 1.0 N HCl, and concentrated to approximately one-half volume (see Note 1).
The mixture is frozen and lyophilized, and the resulting dry solid is purified by silica gel chromatography (CHCl3/MeOH/water/AcOH, 15:9:2:1). The major product corresponding to the main spot observed by TLC is isolated by concentrating the desired fractions.
The resulting solid is dried by azeotropic removal of solvents with toluene (2 × 20 mL) and lyophilized in MeCN/water (1:1) (3 × 15 mL) (see Note 2) to provide a mixture of prosapogenins (5:6, 2.5:1) as a light tan foam (~0.55 g, 50 % mass yield). These xylose and rhamnose-containing prosapogenins 5 and 6 (Fig. 3), respectively, correspond to the two most abundant trisaccharide–triterpene fragments found in QS saponins, and are advanced to the next protection step without further purification.
3.2.2 Synthesis of Triethylsilyl (TES)-Protected Prosapogenin 7 (Fig. 3) by Selective Protection of Prosapogenin Hydroxyl Groups
In a 25-mL modified Schlenk flask, the solid mixture of prosapogenins 5 and 6 (~0.55 g) is azeotroped from pyridine (5 mL), then additional pyridine (8 mL) is added, followed by TESOTf (2.0 mL, 8.8 mmol).
The reaction mixture is stirred for 2.75 days, then TESOTf (0.3 mL, 1.3 mmol) is added, followed by two further additions (0.1 mL each, 0.44 mmol each) 24 h and 48 h later, respectively (see Note 3).
After a total of 5 days, the mixture is concentrated and passed through a short plug of silica gel eluted with hexanes/EtOAc (4:1 to 2:1). The eluate is concentrated, the resulting yellow oil is dissolved in MeOH/THF (1:1) (20 mL), and the solution is stirred for 3.5 days to remove the silyl esters by solvolysis.
The reaction mixture is concentrated and the resulting mixture of xylose- and rhamnose-containing (TES)9-protected prosapogenin diacids is separated by silica gel chromatography (hexanes/EtOAc, 4:1 to 2:1) to afford purified xylose-containing protected prosapogenin 7 (~0.25 g, ~22 % yield) as a white solid.
3.2.3 Synthesis of Protected Quillaja Prosapogenin 8 (Fig. 3) by Selective Esterification of Glucuronic Acid Carboxylic Acid in Protected Prosapogenin 7
In a 10-mL modified Schlenk flask, the prosapogenin diacid 7 (81 mg, 41 μmol, 1.0 equiv.) is dissolved in DCM (0.7 mL) and pyridine (30 μL, 0.37 mmol, 9.0 equiv.) and TBP (102 mg, 0.41 mmol, 10 equiv.) are added, followed by benzyl chloroformate (15 μL, 0.11 mmol, 2.6 equiv.).
The reaction is stirred for 6 h, additional benzyl chloroformate (3.0 μL, 21 μmol, 0.51 equiv.) is added (see Note 4) and the reaction is stirred for another 20 h.
The mixture is concentrated and purified by silica gel chromatography (hexanes/EtOAc, 20:1 to 7:1) to afford selectively glucuronate-protected prosapogenin (8 in Fig. 3) (58 mg, 68 %) as a white solid.
3.3 Isolation and Selective Protection of Quillaic Acid Triterpene, Lacking the Branched Trisaccharide Domain
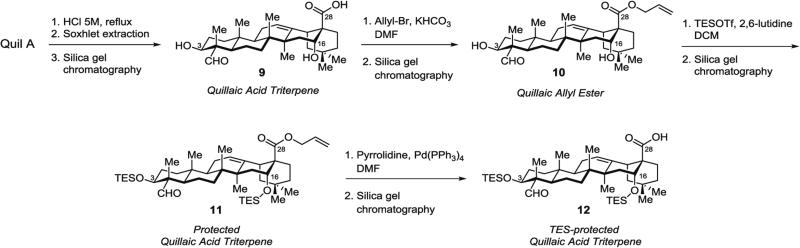
3.3.1 Isolation of Quillaic Acid Triterpene 9 (Fig. 4) from Quil A
Fig. 4.
Isolation and selective protection of quillaic acid triterpene lacking branched trisaccharide domain
In a 250-mL round-bottomed flask equipped with a reflux condenser, Quil A (5 g) is suspended in distilled water (25 mL) and concentrated HCl (17 mL) is added.
The mixture is slowly heated to reflux for 7 h (see Note 5), then removed from heat, and filtered through filter paper. The dark brown solid is washed with hot (~65 °C) distilled water (2 × 50 mL), collected and dried under high vacuum overnight.
The dry solid is placed into a Soxhlet thimble and subjected to continuous extraction with diethyl ether (200 mL) for 24 h.
The ether solution is concentrated, the residue is dissolved in MeOH (20 mL), and activated charcoal (~5 g) is added. The mixture is filtered through celite, the solids are washed with MeOH (50 mL), and the solvent is removed by rotary evaporation.
The resulting residue is purified by silica gel chromatography (CHCl3/MeOH, 30:1 to 20:1 to 10:1) to afford the quillaic acid triterpene 9 (~0.5 g, ~10 % mass yield) (see Note 6).
3.3.2 Synthesis of Quillaic Acid Allyl Ester 10 (Fig. 4) by Allylation of C28 Carboxylic Acid of Quillaic Acid 9
In a 50-mL round-bottomed flask, the quillaic acid triterpene 9 (100 mg, 0.20 mmol, 1.0 equiv.) is dissolved in DMF (5 mL) and the solution is cooled to 0 °C.
Potassium bicarbonate (205 mg, 2.05 mmol, 10 equiv.) and allyl bromide (23 μL, 0.27 mmol, 1.3 equiv.) are added and the mixture is stirred and allowed to warm to room temperature (rt) overnight.
The reaction is diluted with water (25 mL) and extracted with hexanes/EtOAc (1:1) (3 × 15 mL). The organic extracts are combined, washed with brine (15 mL), dried over anhydrous Na2SO4, filtered, and concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 8:1 to 2:1) affords quillaic acid allyl ester 10 (77 mg, 71 %) as a white solid.
3.3.3 Synthesis of Protected Quillaic Acid Triterpene 11 (Fig. 4) by Silylation of C3 and C16 Hydroxyl Groups of Quillaic Acid Allyl Ester 10
In a 25-mL modified Schlenk flask, quillaic allyl ester 10 (77 mg, 0.15 mmol, 1.0 equiv.) is dissolved in DCM (5 mL) and the solution is cooled to 0 °C. 2,6-Lutidine (0.17 mL, 1.46 mmol, 10 equiv.) is added, followed by TESOTf (0.17 mL, 0.73 mmol, 5.0 equiv.) via gas-tight syringe, and the mixture is stirred while the ice bath is allowed to melt.
The reaction progress is monitored by TLC using CHCl3/MeOH (10:1) as eluent. If the reaction is not complete after 3 h, more TESOTf (33 μL, 0.15 mmol, 1.0 equiv.) is added and the mixture is stirred until the reaction is complete.
The reaction mixture is diluted with water (10 mL) and the aqueous phase is extracted with EtOAc (10 mL × 3). The combined organic phases are dried (anhydrous Na2SO4), filtered, and concentrated.
Purification by silica gel chromatography (hexanes/acetone, 1:0 to 10:1) yields the TES-protected quillaic allyl ester 11 (93 mg, 84 %) as a white solid.
3.3.4 Synthesis of TES-Protected Quillaic Acid Triterpene 12 (Fig. 4) by Deallylation of Protected Quillaic Acid 11
In a 10-mL round-bottomed flask, fully protected quillaic acid 11 (93 mg, 0.12 mmol, 1.0 equiv.) is dissolved in DCM (2 mL) and pyrrolidine (51 μL, 0.61 mmol, 5.0 equiv.) is added, followed by Pd(PPh3)4 (7.0 mg, 0.006 mmol, 0.05 equiv.).
The reaction mixture is stirred for 15 min, then directly subjected to purification by silica gel chromatography (hexanes/EtOAc, 2:1), to afford TES-protected quillaic acid 12 (88 mg, >99 %) as a white solid.
3.4 Synthesis of Truncated Linear Oligosaccharide Domain
3.4.1 Synthesis of Selectively Protected Monosaccharide Precursor 2,3,4-tri-O-Benzyl-d-xylose 15 (Fig. 5) from d-Xylose
Fig. 5.
Selective protection of d-xylose
Step A: Synthesis of 1-O-allyl-d-xylose 13 by selective allylation of d-xylose. In a 500-mL round-bottomed flask, a solution of allyl alcohol (50 mL, 0.74 mol, 9.0 equiv.) and AcCl (12.7 mL, 0.17 mol, 2.1 equiv.) is cooled to −10 °C, then solid d-xylose (12.3 g, 0.08 mol, 1.0 equiv.) is added.
Once all xylose has been added, the cooling bath is removed and the reaction mixture is stirred for 19 h at rt.
Solid NaHCO3 (25 g) is added, the mixture is filtered through a pad of celite, and the volatile materials are removed by rotary evaporation.
The residue is passed through a plug of silica gel eluted with DCM/MeOH (9:1) and the eluate is concentrated to afford the anomeric allyl xylose 13 (11.5 g), which is used in the next step without further purification.
Step B: Synthesis of 1-O-allyl-2,3,4-tri-O-benzyl-d-xylose 14 by benzylation of 1-O-allyl-d-xylose 13. In a 500-mL round-bottomed flask, allyl xylose 13 (11.5 g, 60.5 mmol, 1.0 equiv.) is dissolved in DMF (200 mL), then the solution is cooled to 0 °C. Sodium hydride (60 % dispersion in oil, 15.7 g, 0.39 mol, 6.5 equiv.) (see Note 7) is added and the reaction mixture is stirred for 10 min.
Benzyl bromide (47 mL, 0.39 mol, 6.5 equiv.) is added dropwise at 0 °C, and the resulting suspension is stirred at rt for 16 h.
The reaction mixture is cooled to 0 °C and quenched by slow addition of MeOH (150 mL) followed by water (600 mL). The mixture is extracted with hexanes/EtOAc (1:1) (3 × 250 mL) and the combined organic layers are washed with water (100 mL), brine (100 mL), dried with anhydrous MgSO4, filtered, and concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 9:1) affords the fully protected xylose 14 (23 g, 83 %).
Step C: Synthesis of selectively protected 2,3,4-tri-O-benzyl-d-xylose 15 by deallylation of 1-O-allyl-2,3,4-tri-O-benzyl-d-xylose 14. In a 100-mL round-bottomed flask covered in aluminum foil, PPh3 (3.4 g, 13 mmol, 1.2 equiv.) and Pd(OAc)2 (0.45 g, 2.2 mmol, 0.2 equiv.) are dissolved in DCM/MeOH (1:1) (20 mL), then Et2NH (15.8 mL, 0.15 mol, 14.0 equiv.) is added.
A solution of the fully protected xylose 14 (5.0 g, 10.9 mmol, 1.0 equiv.) in DCM (100 mL) is added by cannula transfer, and the reaction mixture is stirred at 30 °C for 18 h.
The solution is passed through a plug of silica gel eluted with hexanes/EtOAc (1:1) and the eluate is concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 8:2 to 7:3) affords 2,3,4,-tri-O-benzyl xylose 15 (4.1 g, 90 %) as a mixture of anomers (α:β, 2:1).
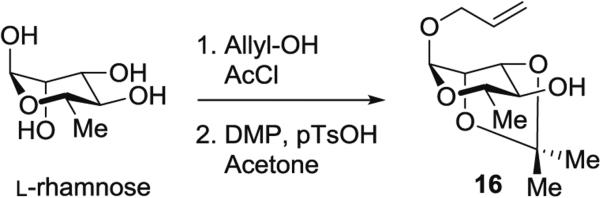
3.4.2 Synthesis of Selectively Protected Monosaccharide Precursor 1-O-Allyl-2,3-O-isopropylidene-l-rhamnose 16 (Fig. 6) from l-Rhamnose
Fig. 6.
Selective protection of l-rhamnose
In a 250-mL round-bottomed flask, a solution of allyl alcohol (34 mL, 0.50 mol, 9.0 equiv.) and AcCl (8.1 mL, 0.12 mol, 2.1 equiv.) is cooled at −10 °C, then l-rhamnose monohydrate (10 g, 0.055 mol, 1.0 equiv.) is added.
The mixture is stirred for 20 h at rt, neutralized with Et3N, and concentrated.
The residue is dissolved in toluene and the solution is concentrated to remove allyl alcohol; this process is repeated two more times.
The residual syrup is dissolved in dry acetone (75 mL), and DMP (27 mL, 0.22 mol, 4.0 equiv.) and pTsOH monohydrate (95 mg, 0.5 mmol, 0.01 equiv.) are added.
The reaction mixture is stirred for 16 h at rt and Et3N is then added.
The reaction mixture is concentrated and purified by silica gel chromatography (hexanes/EtOAc, 8:2) to afford 1-O-allyl-2,3-O-isopropylidene-α-l-rhamnose (16) (8.9 g, 66 %) as a colorless oil.
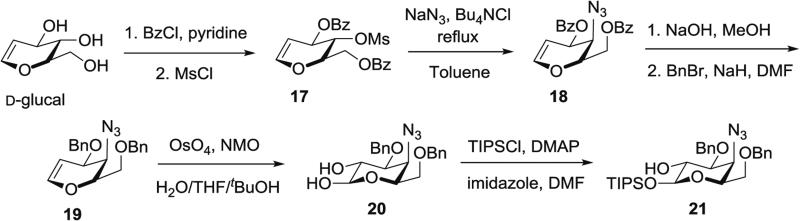
3.4.3 Synthesis of Selectively Protected Monosaccharide Precursor 4-Azido-4-deoxy-3, 6-di-O-benzyl-1-O-triisopropylsilyl-d-galactose 21 (Fig. 7) from d-Glucal
Fig. 7.
Synthesis of protected 4-azido-4-deoxygalactose (21) from d-glucal
Step A: Synthesis of 3,6-di-O-benzoyl-4-O-mesyl-d-glucal 17 by selective protection of d-glucal. In a 500-mL round-bottomed flask, d-glucal (10.0 g, 67.1 mmol, 1.0 equiv.) is dissolved in pyridine (165 mL) and the solution is cooled to 0 °C, then BzCl (17 mL, 0.15 mol, 2.2 equiv.) is added dropwise.
The reaction mixture is stirred at 0 °C for 1.5 h, then MsCl (10.3 mL, 0.13 mol, 2.0 equiv.) is added. The reaction mixture is stirred for 0.5 h while allowing the ice bath warm to rt, then quenched by slow addition of MeOH (20 mL) at 0 °C (see Note 8).
The mixture is concentrated and the residue is partitioned between EtOAc (200 mL) and water (200 mL). The organic layer is washed with water (100 mL), brine (100 mL), dried with anhydrous MgSO4, filtered, and concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 8:2) affords 3,6-di-O-benzoyl-4-O-mesyl-d-glucal (17) (19.4 g, 67 %) as a syrup.
Step B: Synthesis of 4-azido-4-deoxy-3,6-di-O-benzoyl-d-galactal 18 by azide substitution of mesylate 17. In a 250 mL round-bottomed flask, the mesyl-glucal 17 (5.1 g, 11.8 mmol, 1.0 equiv.) is dissolved in toluene (55 mL), then sodium azide (see Note 9) (2.8 g, 43.3 mmol, 3.7 equiv.) is added, followed by Bu4NCl (7.1 g, 25.6 mmol, 2.2 equiv.), and the flask is equipped with a reflux condenser.
The reaction mixture is heated to reflux (110 °C) for 20 h. The resulting brown suspension is washed with water (2 × 100 mL), dried with anhydrous MgSO4, filtered, and concentrated to give an orange oil.
Purification by silica gel chromatography (hexanes/EtOAc, 19:1 to 8:2) provides 4-azido-4-deoxy-3,6-di-O-benzoyl-d-galactal (18) (2.9 g, 66 %) as a light yellow oil.
Step C: Synthesis of 4-azido-4-deoxy-3,6-di-O-benzyl-d-galactal 19 by saponification and benzylation of dibenzoate 18. In 250-mL round-bottomed flask, the benzoyl-protected azidogalactal 18 (2.9 g, 8.1 mmol, 1.0 equiv.) is dissolved in MeOH (40 mL) and the solution is cooled to 0 °C.
Sodium hydroxide (0.12 g, 2.9 mmol, 0.36 equiv.) is added and the reaction mixture is stirred for 14 h at rt.
The reaction mixture is concentrated to afford a sticky tan solid, then evaporated again from toluene (7 mL) to remove trace solvent.
DMF (40 mL) is added to the residue and the resulting brown suspension is cooled to 0 °C. Sodium hydride (60 % dispersion in mineral oil, 0.98 g, 24.4 mmol, 3.0 equiv.) (see Note 7) is added, followed by benzyl bromide (4.8 mL, 40.3 mmol, 5.0 equiv.), and the mixture is stirred at 0 °C for 3 h.
The resulting orange suspension is stirred for another 16 h at rt, and the reaction is quenched with MeOH (20 mL), diluted with DCM (100 mL), and washed with water (100 mL).
The aqueous layer is extracted with DCM (80 mL), and the combined organic layers are washed with water (100 mL), dried with anhydrous MgSO4, filtered, and concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 9:1 to 4:1) affords 4-azido-4-deoxy-3,6-di-O-benzyl-d-galactal (19) (2.2 g, 78 %) as a yellow oil.
Step D: Synthesis of 4-azido-4-deoxy-3,6-di-O-benzyl-d-galactose 20 by dihydroxylation of galactal 19. The benzyl-protected azidogalactal 19 (5.8 g, 16.5 mmol, 1.0 equiv.) is dissolved in a mixture of water/THF/tBuOH (1:3:7) (400 mL), then OsO4 (2.5 wt% in tBuOH) (5.1 mL, 0.4 mmol, 0.025 equiv.) is added. NMO (50 % in water) (10.2 mL, 44.5 mmol, 3.0 equiv.) is added in three portions (1.0 equiv. each) over 8 h.
The reaction mixture is stirred at rt overnight, then quenched with saturated aqueous Na2SO3 solution (30 mL) and EtOAc (200 mL).
After 5 min, the phases are separated and the aqueous layer is extracted with EtOAc (2 × 75 mL) and DCM (2 × 50 mL). The combined organic phases are dried over anhydrous sodium sulfate, filtered, and concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 4:1 to 1:1) affords 4-azido-4-deoxy-3,6-di-O-benzyl-d-galactose (20) (5.5 g, 88 %) as a colorless oil.
Step E: Synthesis of 4-azido-4-deoxy-3,6-di-O-benzyl-1-O-triisopropylsilyl-d-galactose 21 by selective silylation of diol 20. In a 10-mL modified Schlenk flask, the galactose diol 20 (0.96 g, 2.5 mmol, 1.0 equiv.) is dissolved in DMF (2.5 mL), then imidazole (0.41 g, 6.0 mmol, 2.4 equiv.) and DMAP (29 mg, 0.24 mmol, 0.1 equiv.) are added.
TIPSCl (0.63 mL, 3.0 mmol, 1.2 equiv.) is added and the reaction mixture is stirred for 19 h at rt.
The yellow solution is concentrated and purified by silica gel chromatography (hexanes/EtOAc, 19:1 to 9:1) to afford 4-azido-4-deoxy-3,6-di-O-benzyl-1-O-triisopropylsilyl-d-galactose (21) (0.8 g, 59 %) as a colorless oil.
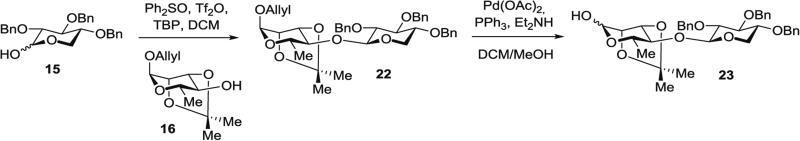
3.4.4 Synthesis of Protected Xylose–Rhamnose Disaccharide Hemiacetal 23 (Fig. 8) ([2,3,4-Tri-O-benzyl-β-d-xylopyranosyl-(1 → 4)]-2,3-di-O-isopropylidene-l-rhamnopyranose) from protected d-xylose 15 and protected l-rhamnose 16
Fig. 8.
Synthesis of protected xylose–rhamnose disaccharide hemiacetal 23 ([2,3,4-tri-O-benzyl-β-d-xylopyranosyl-(1 → 4)]-2,3-O-isopropylidene-l-rhamnopyranose)
Step A: Dehydrative glycosylation of protected rhamnose 16 with protected xylose 15 (22 in Fig. 8): In a 25-mL modified Schlenk flask, azeotropically dried 2,3,4-tri-O-benzyl xylose (15) (52 mg, 0.12 mmol, 1.7 equiv.), Ph2SO (69 mg, 0.34 mmol, 4.9 equiv.), and TBP (85 mg, 0.34 mmol, 4.9 equiv.) are dissolved in DCM (2 mL), injected via glass syringe.
The solution is cooled to −78 °C, Tf2O (29 μL, 0.17 mmol, 2.4 equiv.) is added via gas-tight syringe, and the reaction mixture is stirred for 2 h at −78 °C.
A precooled solution of protected rhamnose 16 (17 mg, 70 μmol, 1.0 equiv.) in toluene (1 mL) is then cannula transferred from a flame dried, 10-mL modified Schlenk flask, then additional toluene (1 mL) is added to rinse the source flask and transferred to the reaction flask.
The reaction mixture is stirred at −60 °C for 12 h, at −42 °C for 30 min, and finally at 0 °C for 2 min.
The reaction is quenched by addition of Et3N (0.1 mL) at −42 °C, diluted with DCM (90 mL) and transferred to a separatory funnel. The organic layer is washed with saturated aqueous NaHCO3 solution (30 mL) and the aqueous layer is extracted with DCM (2 × 80 mL). The organic phases are combined, dried over anhydrous Na2SO4, filtered, and concentrated to afford the crude product as a tan oil (160 mg).
Purification by silica gel chromatography (hexanes/EtOAc, 50:1 to 25:1) affords O-allyl [2,3,4-tri-O-benzyl-β-d-xylopyranosyl-(1 → 4)]-2,3-O-isopropylidene-l-rhamnopyranoside (22) as a clear oil (32.1 mg, 71 % yield).
Step B: Anomeric deallylation of protected xylose–rhamnose disaccharide (23 in Fig. 8): In a 5-mL pear-shaped Schlenk flask equipped with a triangular stir bar, PPh3 (13 mg, 51 μmol, 1.2 equiv.) and Pd(OAc)2 (2.4 mg, 11 μmol, 0.25 equiv.) are placed. A solution of DCM/MeOH (1:1) (0.2 mL) is added via syringe followed by Et2NH (62 μL, 0.6 mmol, 14.0 equiv.), which results in a change from a clear yellow-orange to a bright yellow solution.
Allyl-protected disaccharide 22 (29 mg, 43 μmol, 1.0 equiv.) dissolved in DCM (0.4 mL) is cannula transferred to the reaction Schlenk flask and the source flask is rinsed with additional DCM (0.2 mL) that is transferred to the reaction flask.
The solution is degassed by performing three freeze-thaw-pump cycles (see Note 10) and then stirred at 30 °C for 18 h, at which point the turbid solution turns clear, dark yellow.
The reaction mixture is passed through a plug of silica gel eluted with hexanes/EtOAc (2:1, 50 mL) and the eluate is concentrated to afford the crude product as a bright yellow oil (29 mg).
Purification by silica gel chromatography (hexanes/EtOAc, 2:1) affords disaccharide hemiacetal (23 in Fig. 8) as an inseparable mixture of anomers (α:β, 9:1) as a clear oil (25.9 mg, >99 %).
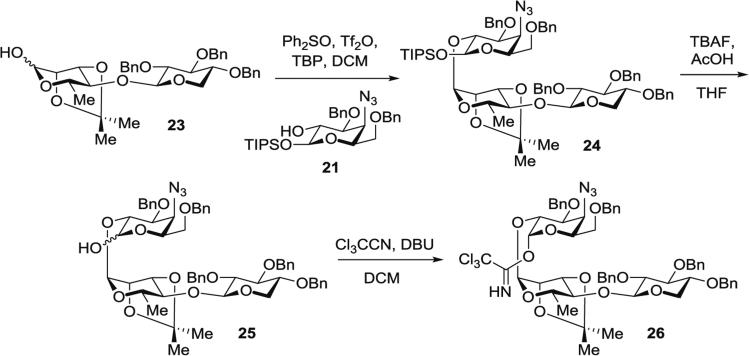
3.4.5 Synthesis of Protected Xylose–Rhamnose–Azidogalactose Trisaccharide Imidate (26 in Fig. 9) (O-Trichloroacetimidoyl {[2,3,4-tri-O-Benzyl-β-d-xylopyranosyl-(1 → 4)]-2,3-O-isopropylidene-l-rhamnopyranosyl-(1 → 2)}-4-azido-4-deoxy-3,6-O-benzyl-β-d-galactopyranoside)
Fig. 9.
Synthesis of protected xylose–rhamnose–azidogalactose trisaccharide imidate 26 (O-trichloroacetimidoyl {[2,3,4-tri-O-benzyl-β-d-xylopyranosyl-(1 → 4)]-2,3-O-isopropylidene-l-rhamnopyranosyl-(1 → 2)}-4-azido-4-deoxy-3,6-di-O-benzyl-β-d-galactopyranoside)
Step A: Synthesis of protected xylose–rhamnose–azidogalactose trisaccharide 24 by dehydrative glycosylation of protected 4-azido-4-deoxygalactose 21 with protected xylose–rhamnose disaccharide 23 (24 in Fig. 9): In a 25-mL modified Schlenk flask, Ph2SO (171 mg, 0.85 mmol, 3.2 equiv.) is dissolved in DCM (3.2 mL). To this clear, colorless solution, Tf2O (76 μL, 0.45 mmol, 1.7 equiv.) is injected via gas-tight syringe at −78 °C. After 10 s, the solution turns pink, then purple, and quickly dissipates back to a clear, colorless solution.
A precooled solution of azeotropically dried disaccharide hemiacetal 23 (185 mg, 0.30 mmol, 1.1 equiv.) in DCM (1 mL) is added to the reaction mixture at −42 °C via cannula from a flame-dried, 5-mL pear-shaped Schlenk flask; then additional DCM (1 mL) is added to rinse the source flask and transferred to the reaction flask.
The reaction mixture is stirred at −42 °C for 15 min, then TBP (190 mg, 0.77 mmol, 3.0 equiv.) is added, and the mixture is further stirred at −42 °C for 1 h.
A precooled solution of protected 4-azido-4-deoxygalactose 21 (141 mg, 0.26 mmol, 1.0 equiv.) in DCM (1 mL) is added to the reaction mixture via cannula from a flame-dried, 5-mL pear-shaped Schlenk flask, at which point white fumes develop. Additional DCM (1 mL) is added to rinse the source flask and transferred to the reaction flask.
The reaction mixture is stirred at −42 °C for 16.5 h and at 0 °C for 1 h, then concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 99:1 to 50:1 to 6:1) gives a mixture of monosaccharide starting material (21) and trisaccharide product (24) as a yellow oil (460 mg). Additional purification of this mixture by silica gel chromatography (hexanes/EtOAc, 10:1 to 6:1) provides the protected trisaccharide 24 (231 mg, 79 %) as a clear oil.
Step B: Synthesis of trisaccharide hemiacetal 25 by anomeric desilylation of protected xylose–rhamnose–azidogalactose trisaccharide 24. In a 250-mL modified Schlenk flask, the protected trisaccharide 24 (575 mg, 0.51 mmol, 1.0 equiv.) is dissolved in THF (50 mL) and the solution is cooled to 0 °C.
A precooled (0 °C) solution of commercially available TBAF (1 M in THF) (0.76 mL, 0.76 mmol, 1.5 equiv.) and AcOH (35 μL, 0.61 mmol, 1.2 equiv.) in THF (50 mL) is added dropwise via cannula to the reaction flask over 50 min at 0 °C.
The reaction mixture is stirred for an additional 5 min at 0 °C, then quenched by addition of saturated aqueous NaHCO3 solution (20 mL).
The contents are transferred to a separatory funnel, EtOAc (125 mL) and brine (50 mL) are added, and the organic phase is separated. The aqueous layer is extracted with EtOAc (2 × 200 mL) and the combined organic phases are dried over anhydrous magnesium sulfate, filtered, and concentrated.
The resulting oil is passed through a plug of silica gel eluted with EtOAc, and the eluate is concentrated to afford the trisaccharide hemiacetal 25 (402 mg, 82 %) as a white foam, which is taken directly to the next step without further purification.
Step C: Synthesis of protected xylose–rhamnose–azidogalactose trisaccharide trichloroacetimidate 26 by activation of protected xylose–rhamnose–azidogalactose trisaccharide 25. In a 100-mL round-bottomed flask, the hemiacetal 25 (200 mg, 0.21 mmol, 1.0 equiv.) is dissolved in DCM (32 mL) and the solution is cooled to 0 °C.
Cl3CCN (0.32 mL, 3.2 mmol, 1.6 equiv.) is added followed by DBU (0.1 mL, 0.67 mmol, 3.3 equiv.) and the reaction is allowed to warm to rt.
After stirring for 13.5 h, the mixture is concentrated to afford an oil.
Purification by silica gel chromatography (hexanes/EtOAc, 6:1 with 0.5 vol% Et3N) (see Note 11) affords the linear trisaccharide imidate 26 (230 mg, >99 %) as a yellow foam.
3.5 Modular, Convergent Assembly of Saponin Domain Fragments
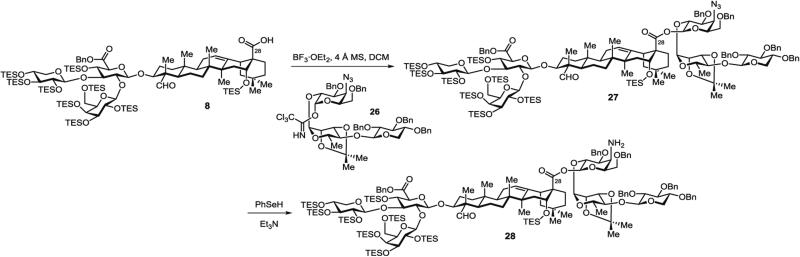
3.5.1 Synthesis of Protected Aminogalactose Saponin 28 (Fig. 10) by Glycosylation of Prosapogenin 8 with Linear Trisaccharide 26 and Azide Reduction
Fig. 10.
Glycosylation of prosapogenin 8 with linear trisaccharide 26 and azide reduction (28)
Step A: Synthesis of protected azidogalactose saponin 27 by glycosylation of branched trisaccharide–triterpene prosapogenin 8 with protected xylose–rhamnose–azidogalactose linear trisaccharide 26. In a 50-mL modified Schlenk flask, the selectively protected prosapogenin 8 (653 mg, 0.32 mmol, 1.5 equiv.) and the trisaccharide imidate 26 (230 mg, 0.21 mmol, 1.0 equiv.) are azeotropically dried from toluene (3 × 3 mL) under high vacuum, then dissolved in DCM (10 mL).
Powdered 4 Å MS (1 g) is added and the suspension is stirred for 2 h at rt. The opaque, white mixture is then cooled to −78 °C and freshly distilled BF3·OEt2 (15 μL, 0.23 mmol, 1.1 equiv.) is injected via gas-tight syringe.
The reaction mixture is stirred at −78 °C for 6 h, passed through a plug of silica gel, and the filtrate is concentrated.
Purification by silica gel chromatography (hexanes/EtOAc, 9:1 to 4:1) affords the prosapogenin – linear trisaccharide conjugate 27 (322 mg, 73 %) as a glassy solid.
Step B: Synthesis of protected aminogalactose saponin 28 by reduction of protected azidogalactose saponin 27. In a 50-mL modified Schlenk flask, PhSeSePh (313 mg, 1.0 mmol, 1.0 equiv.) (see Note 12) is dissolved in THF (8 mL) and H3PO2 (50 % in water) (1.2 mL, 11.0 mmol, 11 equiv.) is then added via syringe.
The yellow solution is heated at 40 °C for 1 h until it turns colorless.
The reaction mixture is removed from the heat, diluted with benzene (8 mL) and distilled water (8 mL), and stirred vigorously for 5 min under Ar. The lower aqueous phase of the resulting biphasic suspension is removed by syringe (or glass pipette) under positive pressure of Ar, and anhydrous sodium sulfate is added to the Schlenk flask to dry the remaining organic layer while stirring.
This freshly prepared solution of PhSeH (~1.9 mmol) is then added under Ar via cannula transfer to a 250-mL reaction Schlenk flask containing a solution of the azeotropically dried saponin azide 27 (322 mg, 0.11 mmol, 1.0 equiv.) in Et3N (50 mL). Upon addition, a white precipitate is formed and the solution turns bright yellow.
The reaction mixture is stirred for 3 h at 40 °C, then concentrated to give a yellow-white solid.
Purification by silica gel chromatography (hexanes/EtOAc, 4:1 to EtOAc with 0.5 vol% Et3N) affords the saponin amine 28 (256 mg, 87 %) as a glassy solid (see Note 13).
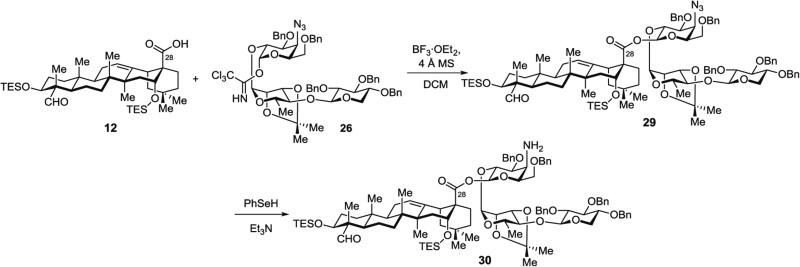
3.5.2 Synthesis of Protected Aminogalactose Saponin 30 (Fig. 11), Lacking the Branched Trisaccharide Domain, by Glycosylation of Protected Quillaic Acid 12 with Linear Trisaccharide 26 and Azide Reduction
Fig. 11.
Glycosylation of protected quillaic acid 12 with linear trisaccharide 26 and azide reduction (30)
Step A: Synthesis of protected azidogalactose saponin 29 by glycosylation of protected quillaic acid 12 with protected xylose–rhamnose–azidogalactose linear trisaccharide 26. In a 25-mL modified Schlenk flask, the selectively protected quillaic acid triterpene 12 (38 mg, 49 μmol, 1.05 equiv.) and the trisaccharide imidate 26 (52 mg, 47 μmol, 1.0 equiv.) are azeotroped from toluene (3 × 1 mL) under high vacuum, then dissolved in DCM (7 mL) and powdered 4 Å MS (80 mg) is added to the solution.
The mixture is stirred for 30 min at rt, then cooled to −42 °C. Freshly distilled BF3·OEt2 (1.2 μL, 9.0 μmol, 0.2 equiv.) is injected via gas-tight syringe and the reaction mixture is stirred for another 30 min at −42 °C.
The reaction is quenched by addition of Et3N (0.2 mL) and the mixture is concentrated by rotary evaporation.
Purification by silica gel chromatography (benzene with 0.5 vol% Et3N to benzene/EtOAc, 97:3) affords the triterpene – linear trisaccharide conjugate 29 (56 mg, 72 %) as a white solid.
Step B: Synthesis of protected aminogalactose saponin 30 by reduction of protected azidogalactose saponin 29. In a 50-mL modified Schlenk flask, PhSeSePh (187 mg, 0.6 mmol, 1.0 equiv.) is dissolved in THF (6 mL) and H3PO2 (50 % in water) (0.72 mL, 6.6 mmol, 11 equiv.) is added via syringe.
The yellow solution is heated at 40 °C for 1 h until it turns colorless.
The reaction mixture is removed from the heat, diluted with benzene (6 mL) and distilled water (6 mL), and stirred vigorously for 5 min under Ar. The lower aqueous phase of the resulting biphasic suspension is removed by glass pipette and the remaining organic layer is dried over anhydrous sodium sulfate while stirring.
This freshly prepared solution of PhSeH (~1.1 mmol, 30 equiv.) is then cannula transferred under Ar to a 100-mL reaction Schlenk flask containing a solution of the azeotropically dried saponin azide 29 (62 mg, 37 μmol, 1.0 equiv.) in Et3N (28 mL). Upon addition, a white precipitate is formed and the solution becomes bright yellow.
The reaction mixture is stirred for 8 h at 38 °C, then concentrated to afford a yellow-white solid.
Purification by silica gel chromatography (benzene/EtOAc, 90:10 to 85:15) affords the truncated saponin amine 30 (49 mg, 80 %) as a glassy solid.
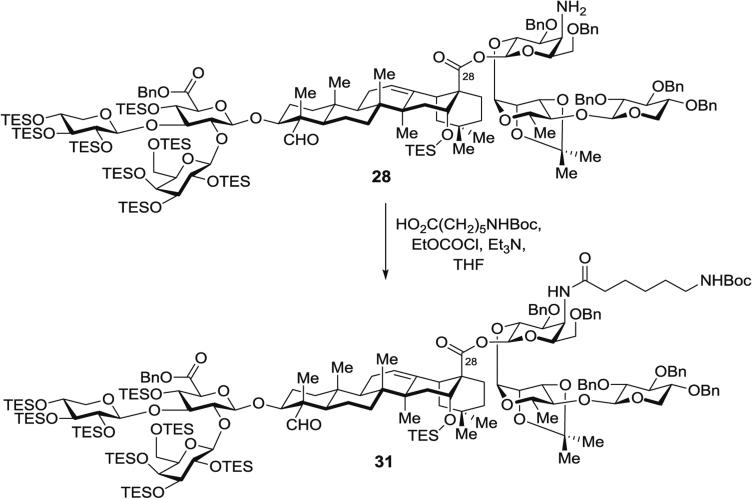
3.5.3 Synthesis of Protected Aminoacyl Saponin 31 (Fig. 12) by Acylation of Protected Aminogalactose Saponin 28
Fig. 12.
Installation of acyl chain domain on aminogalactose residue by acylation of protected branched trisaccharide–triterpene–linear trisaccharide (31)
In a 5-mL pear-shaped Schlenk flask, commercially available 6-(Boc-amino)hexanoic acid (HO2C(CH2)5NHBoc) (19.9 mg, 86 μmol, 10 equiv.) is dissolved in THF (0.9 mL), then Et3N (0.11 mL, 0.77 mmol, 90 equiv.) is added. To this clear, colorless solution at 0 °C, EtOCOCl (7.3 μL, 77 μmol, 9.0 equiv.) is injected via gas-tight syringe.
The turbid white mixture is stirred for 3 h at 0 °C. The prosapogenin – linear trisaccharide saponin amine 28 (26 mg, 8.6 μmol, 1.0 equiv.) is then added, and the reaction is stirred for 1.5 h at rt.
Water (0.1 mL) is added to quench the reaction, at which point the solution turns from turbid white to clear yellow. After addition of more water (0.1 mL), the resulting immiscible mixture is concentrated.
Purification by silica gel chromatography (toluene/EtOAc, 20:1 to 11:1) affords the aminoacyl, branched trisaccharide-containing saponin 31 (22 mg, 81 %) as a white glassy solid.
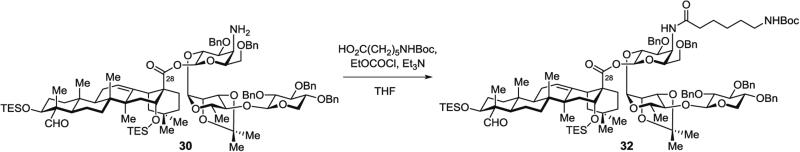
3.5.4 Synthesis of Protected Aminoacyl Saponin 32 (Fig. 13), Lacking the Branched Trisaccharide Domain, by Acylation of Protected Aminogalactose Saponin 30
Fig. 13.
Installation of acyl chain domain on amino galactose residue by acylation of protected triterpene–linear trisaccharide lacking branched trisaccharide domain (32)
In a 10-mL pear-shaped Schlenk flask, 6-(Boc-amino)hexanoic acid (45.0 mg, 0.20 mmol, 11.5 equiv.) is dissolved in THF (2.5 mL), then Et3N (213 μL, 1.53 mmol, 90 equiv.) is added. To this clear, colorless solution at 0 °C, EtOCOCl (16 μL, 0.17 mmol, 10 equiv.) is injected via gas-tight syringe.
The resulting turbid white mixture is stirred for 2.5 h at 0 °C, and then cannula transferred at 0 °C into a 10-mL, Schlenk flask containing a neat film of azeotropically dried (3 × 1 mL toluene) saponin amine 30 (28 mg, 17.0 μmol, 1.0 equiv.).
The turbid white reaction mixture is stirred for 1.5 h at 0 °C, then quenched with water (0.2 mL) to give a clear, colorless solution.
The mixture is diluted with saturated aqueous NaHCO3 solution (30 mL) and the aqueous phase is extracted with DCM (3 × 25 mL). The combined organic layers are dried over anhydrous sodium sulfate, filtered, and concentrated (see Note 14).
Purification by silica gel chromatography (hexanes/EtOAc, 2:1 with 0.5 vol% Et3N) (see Note 15) affords the truncated, fully protected aminoacyl saponin 32 (28 mg, 88 %) as a white glassy solid.
3.6 Global Deprotection of Protected Aminoacylated Saponins
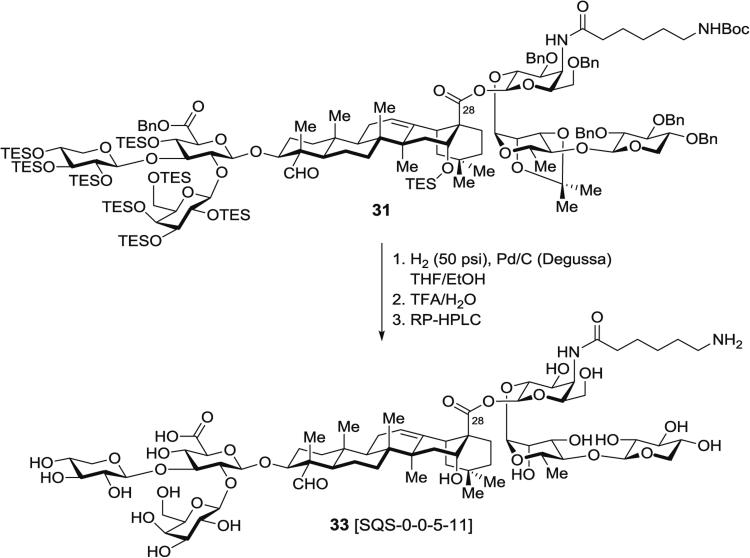
3.6.1 Synthesis of Aminoacyl Saponin 33, SQS-0-0-5-11 (Fig. 14) by Hydrogenolysis and Acid Hydrolysis of Protected Aminoacyl Saponin 31
Fig. 14.
Global deprotection of branched trisaccharide-containing aminoacylated saponin precursor
In a 100-mL round-bottomed flask, fully protected, branched trisaccharide-containing saponin 31 (240 mg, 75 μmol, 1.0 equiv.) is dissolved in THF/EtOH (1:1) (20 mL), then 10 % (dry basis) Pd/C, wet, Degussa type E101 NE/W (140 mg, 66 μmol, 0.9 equiv.) is added (see Note 16).
The reaction mixture is stirred under H2 atmosphere (50 psi) for 24 h at rt using a high-pressure bomb reactor, and the suspension is filtered through a 0.45 μm nylon syringe filter.
The palladium is washed thoroughly with MeOH (3 × 100 mL) and the clear filtrate is concentrated. Successful debenzylation is assessed by disappearance of aromatic resonances by 1H-NMR in methanol-d4.
In a 50-mL round-bottomed flask, the resulting crude mixture of partially desilylated products is dissolved in a precooled (0 °C) solution of TFA/water (4:1) (10 mL).
The reaction mixture is stirred for 3 h at 0 °C and then concentrated under high vacuum at 0 °C to give a white solid residue (140 mg).
This crude product is dissolved in a solution of water/MeCN (4:1) and purified by RP-HPLC using a linear gradient of 20 → 35 % MeCN in water (0.05 vol% TFA) over 10 min. The fraction containing the major, single peak is collected and lyophilized to dryness to afford the fully deprotected, free amine-containing saponin 33 (SQS-0-0-5-11) (88 mg, 78 %) as a fluffy white solid.
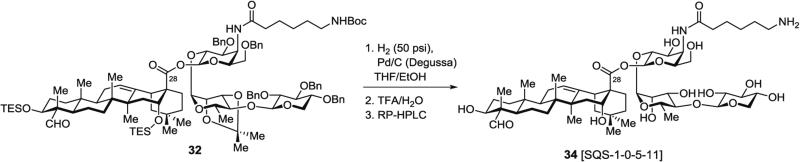
3.6.2 Synthesis of Aminoacyl Saponin 34, SQS-1-0-5-11 (Fig. 15), Lacking the Branched Trisaccharide Domain, by Hydrogenolysis and Acid Hydrolysis of Protected Aminoacyl Saponin 32
Fig. 15.
Global deprotection of truncated aminoacylated saponin precursor lacking branched trisaccharide domain (34)
In a 50-mL round-bottomed flask, the fully protected truncated saponin 32 (68 mg, 36.6 μmol, 1.0 equiv.) is dissolved in THF/EtOH (1:1) (20 mL), then 10 % (dry basis) Pd/C, wet Degussa type E101 NE/W (390 mg, 0.18 mmol, 5.0 equiv.) is added.
The reaction mixture is stirred under an atmosphere of H2 (50 psi) for 24 h at rt using a high-pressure bomb reactor (see Note 17).
The suspension is filtered through a 0.45 μm nylon syringe filter, washed with MeOH (3 × 30 mL) and concentrated. Successful debenzylation is assessed by the disappearance of aromatic resonances by 1H-NMR in methanol-d4.
In a 25-mL round-bottomed flask, the resulting crude mixture is dissolved in a precooled (0 °C) solution of TFA/water (3:1) (8 mL).
The reaction mixture is stirred for 2 h at 0 °C and then concentrated under high vacuum at 0 °C to give a white solid residue.
This crude product is dissolved in water/MeCN (4:1) (20 mL) and purified by RP-HPLC using a linear gradient of 30 → 70 % MeCN in water (0.05 vol% TFA) over 15 min. The fully deprotected, truncated saponin 34 (SQS-1-0-5-11) elutes as a main, single peak and is obtained as a fluffy white solid (28 mg, 74 %) after lyophilization.
3.7 Late-Stage Acylation of Acyl Chain Domain Amine to form Fully Elaborated Saponins 3 (SQS-0-0-5-18) and 4 (SQS-1-0-5-18)
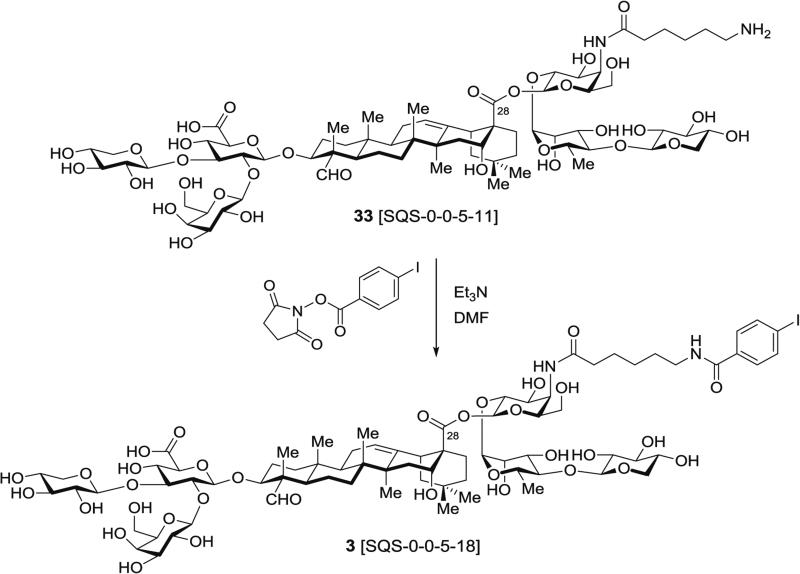
3.7.1 Synthesis of Fully Elaborated Saponin 3, SQS-0-0-5-18 (Fig. 16) by Selective 4-Iodobenzoylation of Free Amine in Aminoacyl Saponin 33
Fig. 16.
4-Iodobenzoylation of free amine in branched trisaccharide–triterpene–linear trisaccharide–acyl chain saponin precursor (3, SQS-0-0-5-18)
In a 10-mL round-bottomed flask equipped with a rubber septum fitted with an Ar inlet needle, amine-terminating saponin 33 (9.0 mg, 6.0 μmol, 1.0 equiv.) is dissolved in DMF (2.0 mL) and Et3N (50 μL, 0.36 mmol, 60 equiv.) is injected via gas-tight syringe.
The mixture is stirred for 50 min at rt and commercially available N-succinimidyl 4-iodobenzoate (20 mg, 60 μmol, 10 equiv.), dissolved in DMF (0.6 mL) under Ar, is then added dropwise via syringe from a 5-mL pear-shaped flask equipped with a rubber septum.
The reaction mixture is stirred for 1 h at rt, diluted with water/MeCN (4:1) (10 mL), and directly purified by RP-HPLC using a linear gradient of 20 → 70 % MeCN in water over 30 min.
The fraction corresponding to the peak containing the desired product, as assessed by mass spectrometry, is collected and lyophilized to dryness to afford the fully elaborated saponin 3 (SQS-0-0-5-18) (5.4 mg, 52 %) as a white powder.
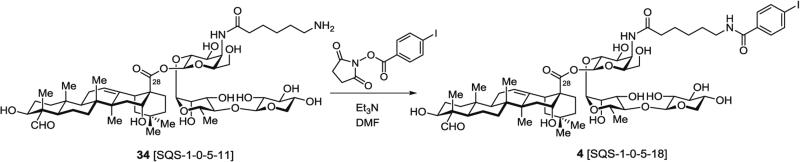
3.7.2 Synthesis of Fully Elaborated Saponin 4, SQS-1-0-5-18 (Fig 17), Lacking the Branched Trisaccharide Domain, by Selective 4-Iodobenzoylation of Free Amine in Aminoacyl Saponin 34
Fig. 17.
4-Iodobenzoylation of free amine in triterpene–linear trisaccharide–acyl chain saponin precursor (4, SQS-1-0-5-18)
In a 5-mL pear-shaped flask equipped with a rubber septum fitted with an Ar inlet needle, amine-terminating truncated saponin 34 (2.1 mg, 2.0 μmol, 1.0 equiv.) is dissolved in DMF (0.4 mL). Et3N (11 μL, 0.08 mmol, 40 equiv.) is injected followed by dropwise addition of a solution of N-succinimidyl 4-iodobenzoate (4.0 mg, 10 μmol, 5.8 equiv.) in DMF (0.2 mL) under Ar via gas-tight syringe.
The reaction mixture is stirred for 2 h at rt, then diluted with 30 % MeCN/water (2.3 mL), and directly purified by RP-HPLC using a linear gradient of 30 → 70 % MeCN in water (0.05 vol% TFA) over 15 min.
The fully elaborated, truncated saponin 4 (SQS-1-0-5-18) (1.7 mg, 67 %) is obtained as a white powder after lyophilization.
Acknowledgements
Dedicated to the memory of our mentor and colleague, Professor David Y. Gin (1967–2011). We thank Dr. George Sukenick, Rong Wang, Dr. Hui Liu, Hui Fang, and Dr. Sylvi Rusli (MSKCC) for expert mass spectral analyses. A. F.-T. thanks the Spanish Ministry of Education (ME–Fulbright postdoctoral fellowship) and the European Commission (Marie Curie Individual Fellowship) for postdoctoral funding. Financial support from the NIH (R01 AI085622 to D. Y. G., R01 GM058833 to D. S. T. and D. Y. G., and P30 CA008748 to C. B. Thompson), William and Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the MSKCC Center for Experimental Therapeutics is gratefully acknowledged.
Footnotes
Care must be taken to avoid excessive foaming and bumping. Water bath should be kept at 35 °C and pressure decreased slowly.
At this point, compare crude 1H-NMR in methanol-d4 with reference spectrum [15]. Since there is significant variability present in the Quillaja bark extracts, some extracts do not contain any of the desired prosapogenin. The best supplier (Brenntag) is mentioned in the Materials section.
The last extra addition of TESOTf is situation-dependent and only required if the reaction is still incomplete after the first 4 days.
The extra addition of CbzCl after the first 6 h depends on the progress of the reaction in each particular case. When purifying by silica gel chromatography, elution with benzene/EtOAc (100:0 to 24:1) can also be considered.
Heating should be done slowly to avoid a foam-over when approaching reflux.
Quillaic acid triterpene product is ~80 % pure. High purity is achieved after allylation reaction.
Caution: sodium hydride reacts violently with water.
Caution: exothermic reaction.
Caution: sodium azide is a toxic, hazardous substance that should not be acidified to avoid poisonous, explosive hydrazoic acid (HN3). The reaction should be carried out behind a blast shield due to risk of explosion of sodium azide when heated near its decomposition temperature (300 °C).
This degassing technique involves freezing the solvent under liquid nitrogen, evacuating the headspace for 4–5 min, and letting the solvent thaw under static vacuum, thereby allowing any gas bubbles trapped in the solvent to escape into the headspace of the flask. After the last cycle, the flask is refilled with Ar.
In absence of Et3N, prolonged chromatography on silica gel when purifying glycosyl trichloroacetimidates leads to progressive hydrolysis of the product.
Caution: selenium compounds are highly toxic and have an unpleasant odor. Phenylselenol itself is extremely noxius. The in situ preparation of phenylselenol solution by reduction of diphenyldiselenide circumvents the need to handle phenylselenol directly, but manipulation of the selenide-containing solution that will be added to the reaction flask is necessary. Care should be taken when handling selenium reagents and all manipulations should be performed in a fumehood wearing protective gloves and safety glasses, including weighing of the diphenyldiselenide starting material. A bleach solution should be prepared in advance to treat all used glassware and possibly early column fractions as well, to oxidize any remaining trace selenides. Bleach solution should also be placed in the solvent trap of the rotary evaporator, which should be thoroughly cleaned after use and ideally contained within the fumehood.
Another alternative experimental procedure to perform this azide reduction step to give the corresponding saponin amine is the treatment of the starting material in Et3N with hydrogen sulfide (gas) as follows. An excess of hydrogen sulfide from a steel cylinder is bubbled via cannula (long steel needle) through an ice-cooled solution of the saponin azide (~45 mg, ~0.015 mmol, 1.0 equiv.) in pyridine/Et3N (3.5:1) (4.5 mL) for 2 min. Vent needle and cannula are removed from septum, which is sealed with Teflon tape and parafilm, and the reaction mixture is stirred overnight at rt. The dark green solution is then purged of excess hydrogen sulfide with a stream of nitrogen, and the resulting light-orange solution is concentrated by rotary evaporation. Purification of the residue by silica gel chromatography (hexanes/EtOAc, 1.0 vol% Et3N) yields the desired saponin amine product (~40 mg, 80–90 % yield).
After quenching the reaction with water, the mixture can also be directly concentrated by rotary evaporation without the need for performing the described aqueous work-up.
Elution with 9:1 to 5:1 benzene/EtOAc (0.5 vol% Et3N) can also be used for the silica gel chromatography purification.
Caution: hydrogenolysis reactions pose a significant fire hazard. Caution should be taken when handling flammable palladium on carbon as well as hydrogen gas, which increases the risk of explosion.
In similar saponin triterpene variants lacking the branched trisaccharide domain, hydrogenolysis under hydrogen atmosphere at balloon pressure for 12 h is sufficient to provide the corresponding debenzylated products.
References
- 1.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 2.Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines. 2011;10:463–470. doi: 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RTS,S Clinical Trials Partnership Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Kim YJ, Navarro-Villalobos M, Rohde BD, Gin DY. Synthesis of the potent immunostimulatory adjuvant QS-21A. J Am Chem Soc. 2005;127:3256–3257. doi: 10.1021/ja0422007. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Wang P, Navarro-Villalobos M, Rohde BD, Derryberry J, Gin DY. Synthetic studies of complex immunostimulants from Quillaja saponaria : synthesis of the potent clinical immunoadjuvant QS-21Aapi. J Am Chem Soc. 2006;128:11906–11915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng K, Adams MM, Damani P, Livingston PO, Ragupathi G, Gin DY. Synthesis of QS-21-xylose: establishment of the immuno-potentiating activity of synthetic QS-21 adjuvant with a melanoma vaccine. Angew Chem Int Ed. 2008;47:6395–6398. doi: 10.1002/anie.200801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng K, Adams MM, Gin DY. Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants. J Am Chem Soc. 2008;130:5860–5861. doi: 10.1021/ja801008m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams MM, Damani P, Perl N, Won A, Hong F, Livingston PO, Ragupathi G, Gin DY. Design and synthesis of potent Quillaja saponin vaccine adjuvants. J Am Chem Soc. 2010;132:1939–1945. doi: 10.1021/ja9082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chea EK, Fernández-Tejada A, Damani P, Adams MM, Gardner JR, Livingston PO, Ragupathi G, Gin DY. Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J Am Chem Soc. 2012;134:13448–13457. doi: 10.1021/ja305121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Tejada A, Chea EK, George C, Pillarsetty N, Gardner JR, Livingston PO, Ragupathi G, Lewis JS, Tan DS, Gin DY. Development of a minimal saponin vaccine adjuvant based on QS-21. Nat Chem. 2014;6:635–643. doi: 10.1038/nchem.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Tejada A, Chea EK, George C, Gardner JR, Livingston PO, Ragupathi G, Tan DS, Gin DY. Design, synthesis, and immunologic evaluation of vaccine adjuvant conjugates based on QS-21 and tucaresol. Bioorg Med Chem. 2014;22:5917–5923. doi: 10.1016/j.bmc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Tejada A, Tan DS, Gin DY. Versatile strategy for the divergent synthesis of linear oligosaccharide domain variants of Quillaja saponin vaccine adjuvants. Chem Commun. 2015;51:13949–13952. doi: 10.1039/c5cc05244k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkowicz WE, Fernández-Tejada A, George C, Corzana F, Jiménez-Barbero J, Ragupathi G, Tan DS, Gin DY. Quillaja saponin variants with central glycosidic linkage modifications exhibit distinct conformations and adjuvant activities. Chem Sci. 2016;7:2371–2380. doi: 10.1039/c5sc02978c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo S, Kenne L, Lundgren LN, Rönnberg B, Sundquist BG. Triterpenoid saponins from Quillaja saponaria. Phytochemistry. 1998;48:175–180. doi: 10.1016/s0031-9422(97)00716-4. [DOI] [PubMed] [Google Scholar]