Fig.1. Schematic illustration of the mechanism of cation-chloride transporters in the brain.
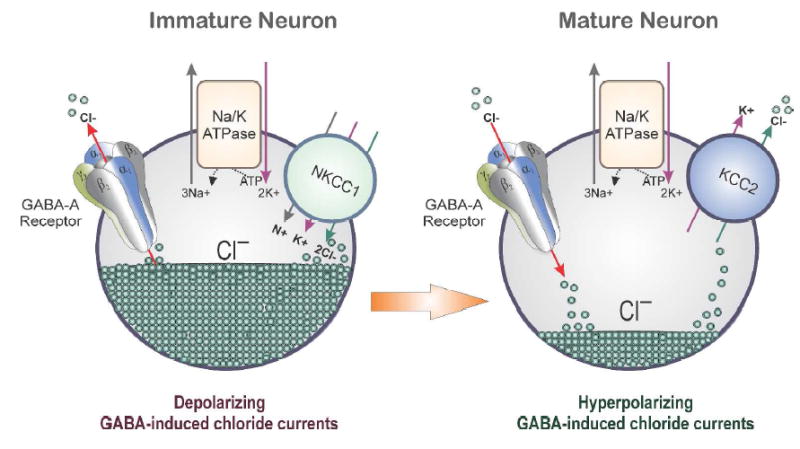
In neurons, KCCs (K—Cl cotransporters) control the reversal potential (E-GABA) of GABA-A receptor-mediated current and voltage responses. Therefore, they influence the efficacy of GABAergic inhibition. The neuron-specific KCC isoform, KCC2, is the main Cl- extruding mechanism in hippocampal and neocortical principal neurons. Activation of GABA-A receptors on the membranes of mature neurons generally results in the influx of Cl− and subsequently membrane hyperpolarization. However, in immature neurons, GABA is depolarizing due to the high intracellular chloride gradient (50 mM). This is attributed to an initially dominant role of Cl− uptake mediated by NKCC1, followed by a developmental up-regulation of the Cl− extruding KCC2, which renders the equilibrium potential of Cl− hyperpolarizing. In adult and immature neurons, the overall chloride gradient actually depends on the relative activity and abundance of NKCC1 and KCC2. Consequently, sex differences in the developmental shift from dominant NKCC1 to dominant KCC2 expression and/or activity may underlie the earlier switch of GABA-A receptor signaling from depolarizing to hyperpolarizing in certain brain regions in the female brain. These changes may ultimately contribute to alteration in GABAergic inhibition and seizure susceptibility.
