Abstract
Studies using the nematode C. elegans have provided unique insights into the development and function of sex differences in the nervous system. Enabled by the relative simplicity of this species, comprehensive studies have solved the complete cellular neuroanatomy of both sexes, as well as the complete neural connectomes of the entire adult hermaphrodite and the adult male tail. This work, together with detailed behavioral studies, has revealed three aspects of sex differences in the nervous system: sex-specific neurons and circuits; circuits with sexually dimorphic synaptic connectivity; and sex differences in the physiology and functions of shared neurons and circuits. At all of these levels, biological sex influences neural development and function through the activity of a well-defined genetic hierarchy that acts throughout the body to translate chromosomal sex into the state of a master autosomal regulator of sexual differentiation, the transcription factor TRA-1A. This review focuses on the role of genetic sex in implementing sex differences in shared neurons and circuits, with an emphasis on linking the sexual modulation of specific neural properties to the specification and optimization of sexually divergent and dimorphic behaviors. An important and unexpected finding from these studies is that chemosensory neurons are a primary focus of sexual modulation, with genetic sex adaptively shaping chemosensory repertoire to guide behavioral choice. Importantly, hormone-independent functions of genetic sex are the principal drivers of all of these sex differences, making nematodes an excellent model for understanding similar but poorly understood mechanisms that likely act throughout the animal kingdom.
Keywords: Caenorhabditis elegans, sexual dimorphism, sex differences, genetic sex, sexual behavior, neural circuits and behavior
INTRODUCTION
With its “simple” neuroanatomy and behavior as well as a powerful experimental toolkit, the nematode C. elegans has provided numerous insights into the genetic mechanisms that specify cell fates and build neural circuits, the neural control of innate behaviors, and the modulatory influences that alter behavior according to an animal’s experience and environment. Indeed, one of Sydney Brenner’s primary motives in establishing C. elegans as a model system in the late 1960s was to bring tractability to fundamental questions about the neural control of behavior (Ankeny 2001). An additional key goal was to understand the genetic control of developmental programs that build a multicellular organism. In this latter vein, some of the earliest work on C. elegans developmental control concerned sex determination, the mechanism by which a simple difference in sex chromosome dosage brings about the multiple concerted developmental changes that distinguish the sexes from each other. At the interface between these two areas is the relationship between chromosomal sex and neurobiology. Hodgkin appreciated the significance of this question, and the potential of C. elegans to address it, 25 years ago (Hodgkin 1991). Work in the 1980s and ‘90s made important progress in this area, linking the chromosomal sex of neural precursors to the alterations in proliferation and differentiation that give rise to sexually dimorphic neuroanatomy; this has been reviewed elsewhere (Emmons 2014; Fagan and Portman 2014; Portman 2007). Here, we consider more recent findings that establish a role of genetic sex to the functional modulation of non-sex-specific (“shared”) neurons and circuits.
Historically, sexual differentiation in the vertebrate (and particularly mammalian) nervous system has not been viewed in the context of genetic sex. Instead, attention has focused on the powerful developmental and physiological roles of gonadal steroids on the brain. According to the simplest view of gonadal hormone function, chromosomal sex would be relevant only in the gonad, where it specifies sexually dimorphic development and physiology of the gonads. The influence of chromosomal sex on the brain would then be completely indirect, relying exclusively on non-autonomous signaling mechanisms. However, multiple lines of evidence in mammals and other vertebrates have clearly demonstrated that gonadal steroids are not the sole influence of biological sex on the nervous system, and that chromosomal sex outside of the gonad, likely in the nervous system itself, has clear roles in neural development and behavior (McCarthy and Arnold 2011). While much about these hormone-independent “genetic” processes remains unclear, these findings indicate that some of the mechanisms that guide sex differences in the vertebrate and invertebrate nervous systems may be more similar than has been previously appreciated. Thus, studies of invertebrate models may provide important insights relevant for mammalian systems.
Work over the past decade has established that genetic sex regulates not only the development of the C. elegans nervous system but also has deep influences on circuit physiology and behavior. This work has provided new insight into the mechanisms by which “cellular sex” can regulate the properties of specific classes of neurons. Moreover, it has allowed the identification of key regulatory nodes that sex uses to adaptively tune circuit function and behavior. This article only briefly addresses the important developmental influences of biological sex; several other reviews examine these issues more thoroughly (Emmons 2014; Fagan and Portman 2014; Portman 2007). Instead, the focus here is on more recent findings concerning the influence of cellular sex on the physiology and function of shared neural circuits.
SEX DETERMINATION IN C. ELEGANS
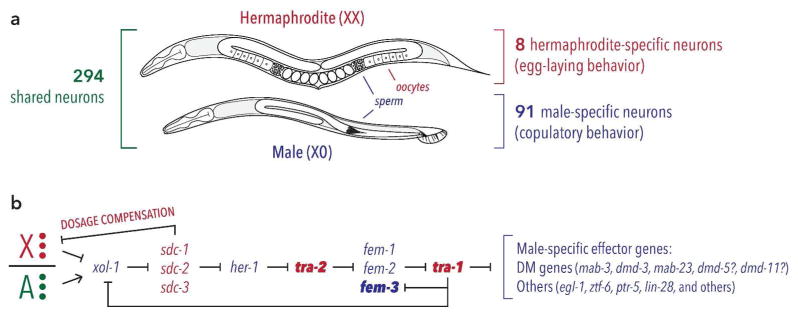
The two sexes of C. elegans do not conform precisely to the standard categories of male and female (Fig. 1a). Instead, XX individuals transiently produce self-sperm before producing oocytes, earning them the designation of hermaphrodite rather than female. Though these animals are indeed somatically female, the gonad is briefly masculinized during development to produce a limited number of sperm that are stored in the spermatheca. At the transition to adulthood, the hermaphrodite’s germline becomes permanently feminized and produces only oocytes for the rest of the animal’s reproductive lifespan. As a hermaphrodite’s oocytes pass through the spermatheca, they are fertilized by its self-sperm, unless the individual has mated with a male, whose sperm tend to be used preferentially. Because the C. elegans hermaphrodite has the body of a female, it lacks male genitalia and therefore cannot mate with other hermaphrodites. Not unexpectedly, these differences in reproductive mode are likely to impact C. elegans behavioral strategies. In particular, males must find mates to reproduce, but hermaphrodites need to find suitable environments in which to lay eggs. As a result, worms display sex differences not only in behaviors that are overtly reproductive (e.g., male mating), but also in features of behavioral programs that are shared by both sexes.
Figure 1. Sexual dimorphism and sex determination in the nematode C. elegans.
(A) Adult males and hermaphrodites share an overall body plan but differ in germline, gonad, genitalia, and many other features. Adults of both sexes possess exactly 294 shared neurons, while adult hermaphrodites and adult males possess 8 and 91 sex-specific neurons, respectively. (B) The genetic hierarchy controlling C. elegans sex determination in the soma is triggered by the ratio of sex chromosomes to autosomes (X/A ratio). Genes active in XX animals are shown in red, while those active in XO animals are shown in blue. The X/A ratio is “calculated” through the molecular activities of dosage-sensitive X- and A-linked genes (red and green circles, respectively), which converge on the regulation of the autosomal gene xol-1. As a result of this regulation, xol-1 is expressed only in XO embryos, where it regulates both sexual differentiation and well as dosage compensation by repressing the sdc genes. Downstream of the sdc genes, dosage compensation and sexual differentiation are implemented through independent pathways. At the terminus of the sex determination pathway lies the “master regulator” tra-1, shown in bold. Acting via its product TRA-1A, this gene is both necessary and sufficient for essentially all sex differences in the soma, including the nervous system. Several known direct targets of TRA-1A are shown, with question marks indicating targets that have been suggested to be direct. TRA-1A also regulates xol-1 and fem-3, providing feedback that likely stabilizes the state of the pathway once dosage compensation equalizes the X-to-A ratio. tra-2 and fem-3, both shown in bold, are normally active in only in hermaphrodites or males, respectively. Forced expression of these genes in specific tissues of the opposite sex is largely sufficient to reverse their sexual state by activating or inhibiting TRA-1A.
C. elegans sex is determined chromosomally, with XX embryos developing into hermaphrodites and X0 embryos becoming males. (The lack of a heteromorphic sex chromosome—in this case, Y—emphasizes the fact that, as in most animals, sex-specific genes are not essential for sexual differentiation. Both sexes share the same genome, suggesting that sex differences come about by the modulation of shared mechanisms.) As a result of this system, nearly all of the self-progeny of a hermaphrodite arise from the fusion of two X-bearing germ cells, resulting in more XX hermaphrodites. A low frequency of X nondisjunction in this process (~0.3%) allows the production of rare X0 males via self-fertilization. When a male fertilizes a hermaphrodite, however, cross-progeny emerge in a 50:50 sex ratio.
A series of classic studies led by Hodgkin and Meyer identified the genetic pathway linking sex chromosome status in C. elegans to the determination of an individual’s phenotypic sex (Fig. 1b) (Wolff and Zarkower 2008). Briefly, a chromosome-counting mechanism takes advantage of specific dosage-sensitive genes on X and the autosomes to measure the X-to-autosome ratio (Gladden et al. 2007). This ratio determines the activity of a critical autosomal gene, xol-1, which is activated only in X0 animals (Rhind et al. 1995). xol-1 has two key functions: first, it represses dosage compensation, allowing this process to occur only in hermaphrodites. Second, it sets the state of a cascade of negative regulatory events that, in the soma, lead to the regulation of the gene tra-1. This autosomal gene, which encodes the Gli-family Zn finger transcription factor TRA-1A (Zarkower and Hodgkin 1992), is the “master regulator” of sex in C. elegans: it is the terminal gene in the pathway that controls all sexually differentiated somatic traits (Hodgkin 1987). In XX embryos, the sex determination pathway activates TRA-1A, while in X0 embryos, TRA-1A function is blocked. Consistent with this, null mutations in tra-1 convert XX animals into fertile males, while gain-of-function tra-1 alleles strongly feminize X0 animals (de Bono et al. 1995; Hodgkin 1987). Notably, tra-1’s function in sex determination is not known to be conserved outside nematodes. This is consistent with findings from many other animals, including insects and mammals, indicating that upstream components of sex-determination hierarchies evolve rapidly (Wilkins 1995; Williams and Carroll 2009). In contrast, some of tra-1’s direct targets are members of the DM domain family, a group of genes whose functions in sex determination and sexual differentiation are conserved across the animal kingdom (Matson and Zarkower 2012). As discussed below, little is known about the roles of DM domain genes in modulating the properties of shared neurons and circuits in C. elegans, but it seems likely that genes of this family carry out this function in nematodes as well as in more complex species.
As a transcription factor, TRA-1A functions cell-autonomously, such that its activity can be considered to encode the sexual state of any given cell (Hunter and Wood 1990). Because TRA-1A is thought to function primarily (if not exclusively) as a transcriptional repressor, its function is to repress the expression of target genes that promote male-specific developmental and physiological events. These targets are thought to be specialized for functions in different tissue types at different stages during development. While several targets of TRA-1A have been identified (Berkseth et al. 2013; Conradt and Horvitz 1999; Hargitai et al. 2009; Mason et al. 2008; Yi et al. 2000), they do not account for the full extent of sexual dimorphism in the C. elegans soma; moreover, the mechanisms by which they implement sex differences remains largely unclear.
THE C. ELEGANS NERVOUS SYSTEM
Several distinctive features make C. elegans an ideal model for studying sex differences in the nervous system. First, the complete cell lineage, from single-celled zygote to sexually mature adult, is known for both sexes (Sulston et al. 1980; Sulston and Horvitz 1977; Sulston et al. 1983). Because these lineages are largely invariant among individuals, this provides a complete catalog of lineally equivalent neurons present in both sexes (294) as well as neurons found only in one sex (8 in hermaphrodites and 91 in males). Second, the complete wiring diagrams (“connectomes”) of the adult hermaphrodite (White et al. 1986), as well as that of the adult male tail (Jarrell et al. 2012), have been determined from analysis of serial electron micrographs. Work on the connectome of the male head is currently in progress (see wormwiring.org). This understanding of circuit-level organization provides a unique inroad to connecting circuit structure and function to behavior.
The most obvious aspect of sex differences in the C. elegans nervous system is the simple presence or absence of sex-specific neurons. In hermaphrodites, these are midbody motor neurons that control vulval muscle contraction and egg-laying behavior (Schafer 2005). In males, sex-specific neurons are present mostly in the tail, where they form circuits subserving the multistep behavioral program of copulation (Jarrell et al. 2012). Developmentally, there are three mechanisms that generate sexual dimorphism at this level: sex-specific programmed cell death, sex-specific alterations in the lineage of precursor cells, and sex-specific neurogenesis. In some cases, it is clear how tra-1 regulates these processes to generate sexual dimorphism, but in most, this remains unknown. These sexually dimorphic features of the nervous system and behavior have been reviewed elsewhere (Emmons 2014; Fagan and Portman 2014; Portman 2007).
SEX DIFFERENCES IN SHARED NEURONS AND CIRCUITS
Superimposed onto the sex-specific components of the C. elegans nervous system are 294 neurons that are lineally equivalent and nearly anatomically identical between hermaphrodites and males. These have been referred to previously as the “core nervous system” (Portman 2007). However, because these neurons are deeply interconnected with sex-specific neurons (Jarrell et al. 2012; White et al. 1986), there is no meaningful distinction between a “core” and a “sex-specific” nervous system. Hence we refer here to these 294 neurons common to both sexes as “shared” neurons.
Because of this apparently high degree of similarity, shared neurons and circuits were historically considered to be functionally equivalent in both sexes. However, multiple studies over the past decade have indicated that the functions of shared neurons and circuits are deeply modulated by sex. Several observations provided important initial support for this idea (Portman 2007). First, gene expression patterns were found to differ between the sexes in two shared neurons, ADF and AIM. In both of these cases, reporter expression (for srd-1 and srj-54, respectively) was observed only in males, despite the presence of ADF and AIM in both sexes (Lee and Portman 2007; Troemel et al. 1995). Recently, AIM has also been shown to undergo a male-specific neurotransmitter identity switch (from glutamatergic to cholinergic) upon sexual maturation (Pereira et al. 2015). Some morphological differences were also known among shared neurons; for example, the ultrastructure of the tail sensory neuron PHC differs by sex (Sulston et al. 1980). Additionally, anecdotal observations indicated that some shared behaviors, particularly locomotion, differ between the sexes (Hodgkin 1974). However, most research on C. elegans neurobiology and behavior have historically focused on the hermaphrodite, and efforts in the male emphasized the multistep behavioral program of copulation.
A key advance in identifying the important roles of genetic sex in shared neurons was the development of strategies to generate “sexually mosaic” animals, individuals in which the sexual state of particular cells or tissues is mismatched with the rest of the organism. As described below, this strategy was first developed by White et al. and Lee et al. in studies of chemosensory behavior (Lee and Portman 2007; White et al. 2007), and has since been extended to a number of developmental and physiological sex differences in the nervous system. Briefly, cell-type-specific expression of the normally male-specific gene fem-3 in the hermaphrodite is sufficient to destabilize TRA-1A and functionally masculinize particular cells or tissues (Lee and Portman 2007; White et al. 2007). Reciprocally, expression of tra-2(ic), a constitutively active form of the hermaphrodite-specific gene tra-2, can stabilize TRA-1A and feminize cells (Mowrey et al. 2014). Expression of a gain-of-function allele of tra-1 has also been used for this purpose (Sakai et al. 2013). As described below, these tools have been used in a variety of contexts, and continue to be an important experimental approach.
BEHAVIORAL PRIORITIZATION: FEEDING VS. EXPLORATION
Aside from sexual behavior, one of the most apparent sex differences in C. elegans behavior is the response to food (Lipton et al. 2004). A small patch of bacterial food will efficiently retain individual adult hermaphrodites: animals will occasionally wander off the patch but nearly always return immediately. An isolated male, however, behaves quite differently. The individual will feed and explore the food patch, but often fails to reverse course when it encounters the edge. In the laboratory, males that leave the food source explore the rest of the agar plate, often fatally, as they tend to climb up the plastic wall and desiccate. This behavior might seem highly maladaptive, but its significance is illustrated by the behavioral change that occurs when a male finds a food spot that harbors a hermaphrodite. The presence of this potential mate is sufficient to completely suppress food-leaving behavior, indicating that food leaving is likely a mate-searching behavior, an exploratory state motivated by sexual drive (Lipton et al. 2004). Several additional lines of evidence are consistent with this idea: a food-deprived male will suppress its food-leaving behavior for several hours, eating until it has recovered from starvation; larval (sexually immature) males almost never leave a food spot; and hermaphrodites that cannot produce self-sperm also exhibit some food-leaving behavior (Lipton et al. 2004).
Several mechanisms regulate this sex difference in the balance between feeding and exploration, and the relationship between these remains unclear. Signals from the gonad, as well as insulin, serotonin, and bile-acid hormone signaling, all modulate male food-leaving behavior (Kleemann et al. 2008; Lipton et al. 2004). The food-leaving drive is also promoted by PDF neuropeptide signaling (Barrios et al. 2012) as well as the activity of male-specific sensory neurons, likely through their tonic activity (Barrios et al. 2008).
In addition to these mechanisms, sex differences in the response to food-derived odorants play an important role in determining the balance between feeding and mate-searching. In studies of olfactory behavior, males and hermaphrodites were found to have distinct preferences for different odorants; in particular, the food-associated odorant diacetyl was significantly more attractive to hermaphrodites than males (Lee and Portman 2007). To test the possible involvement of genetic sex acting in the nervous system, these investigators then selectively expressed fem-3 throughout the nervous system, or specifically in the set of 60 ciliated sensory neurons the worm uses to sense its environment. Both of these manipulations were able to significantly masculinize hermaphrodite olfactory preference (Lee and Portman 2007). Thus, functional modulation of olfaction occurs at least in part through the activity of the sex determination pathway in sensory neurons.
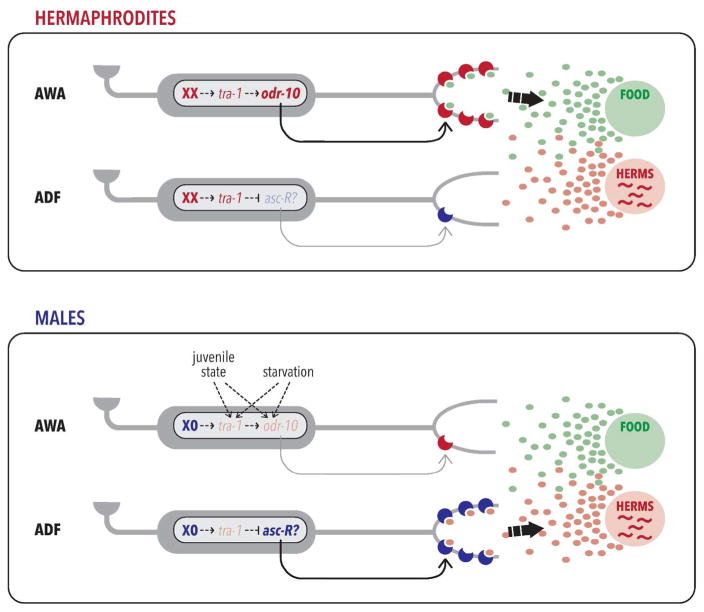
A key mechanism downstream of genetic sex is the regulation of odorant receptor gene expression, providing a simple, elegant mechanism for tuning behavioral output by modulating sensory function. Ryan et al. found that the sex difference in diacetyl attraction was controlled by the genetic sex of a single pair of sensory neurons called AWA: genetically masculinizing just these two neurons in hermaphrodites reduced diacetyl and food attraction, and generated some food-leaving behavior (Ryan et al. 2014). Feminizing these neurons in males increased diacetyl and food attraction and suppressed food-leaving. Moreover, AWA’s genetic sex was found to target the expression level of the GPCR ODR-10, previously shown to be the major chemoreceptor for diacetyl (Sengupta et al. 1996). In adult hermaphrodites, high levels of odr-10 expression in AWA promote attraction to diacetyl and food. However, in adult males, low odr-10 expression reduces male food sensitivity (Fig. 2). Importantly, engineering increased odr-10 expression in adult males is able to increase male food attraction and decrease food-leaving behavior. It also decreases the male’s ability to locate hermaphrodite mates in an environment in which males must choose between feeding and exploration, demonstrating the importance of this mechanism for reproductive success. Finally, food deprivation causes a transient activation of odr-10 expression in males; this promotes the transient re-prioritization of feeding over exploration in response to nutritional state (Ryan et al. 2014).
Figure 2. Sensory repertoires are tuned cell-autonomously by genetic sex.
In hermaphrodites, the genetic sex of the AWA neuron triggers high levels of expression of odr-10, the receptor for the food odorant diacetyl (Ryan et al. 2014). In the ADF neuron and perhaps others, genetic sex acts cell-autonomously to inhibit sensitivity to sex pheromones (Fagan et al., in preparation), potentially also by regulating ascaroside receptor (“asc-R”) expression. The net effect of this is that food cues become more salient for hermaphrodites, promoting the prioritization of feeding behavior. In males, the state of the sex determination pathway is reversed; this inhibits odr-10 expression in AWA and increases the sensitivity of ADF and perhaps other neurons to ascaroside sex pheromones. (Dashed arrows inside the nucleus indicate indirect regulation; for simplicity, the entire pathway is not shown.) Thus, differential perception of chemosensory cues causes males to leave food and search for mates. In starved and larval males, odr-10 expression is upregulated. How these signals converge with genetic sex to regulate odr-10 remains unknown, but the effect of this regulation is to increase male attraction to food.
A number of important questions regarding this mechanism remain open. First, the means by which TRA-1A acts in AWA to regulate odr-10 expression is unknown. This regulation is unlikely to be direct, as there are no obvious binding sites for TRA-1A near odr-10 (Berkseth et al. 2013); moreover, TRA-1A is thought to repress its transcriptional targets, but genetically, tra-1 promotes odr-10 expression in AWA. DM-domain genes (Matson and Zarkower 2012) are good candidates for intermediates that connect TRA-1 to odr-10 expression, though such a role has not yet been described. Related to this issue is the question of whether odr-10 is the sole gene regulated by the genetic sex of AWA, or whether the functional properties of AWA are extensively tuned by sex. As discussed below, another open question regards the mechanism whereby the sex difference in odr-10 expression and AWA function is developmentally controlled. While adults exhibit a clear sex difference in odr-10 expression, larvae of both sexes display equivalent, moderate levels of odr-10 (Ryan et al. 2014). As the sex-chromosome state of AWA is a static signal, unknown mechanism(s) must act to gate its effects on AWA.
SEX-SPECIFIC CHEMOSENSATION: PHEROMONE DETECTION
The finding that adult males suppress their sensitivity to food in order to generate exploration raises the question of whether complimentary changes in chemosensation might allow males to locate mates. Supporting this possibility, several studies have indicated that hermaphrodites produce a variety of secreted cues that act as sex pheromones (Leighton and Sternberg 2016). The existence of such cues was initially demonstrated by Simon and Sternberg (Simon and Sternberg 2002); subsequent work by several groups has indicated that multiple classes of molecules produced by hermaphrodites elicit male-specific attraction (Leighton et al. 2014; Pungaliya et al. 2009; Srinivasan et al. 2008; White et al. 2007). Sex-specific sensory neurons in the male head, the CEMs, play an important role in generating sexually dimorphic responses to at least some of these pheromones (Narayan et al. 2016; Srinivasan et al. 2008; White et al. 2007).
Several lines of evidence indicate that sex-specific tuning of shared chemosensory circuits also contributes to sex pheromone attraction. The first evidence for this arose from studies of male responses to culture medium conditioned by hermaphrodites (White et al. 2007). Although the active component(s) of this preparation are unclear, they are not ascaroside-class pheromones (see below). White et al. found that male attraction to this mixture requires several shared olfactory neuron pairs in the C. elegans head, particularly AWA and AWC, and that pan-neural sex reversal is sufficient to generate attraction in hermaphrodites (White et al. 2007). Subsequent work showed that masculinizing a distributed set of sensory neurons and interneurons including AWA and AWC is necessary for this behavioral effect. Moreover, sex reversal during development, but not in adulthood, can masculinize hermaphrodite behavior in this assay, suggesting a role for genetic sex in the wiring, rather than the physiology, of this circuit. These studies also suggested that a sex-specific role for the TGFβ-superfamily ligand DAF-7 may inhibit pheromone attraction in hermaphrodites (White and Jorgensen 2012). The mechanisms whereby genetic sex influences this behavior remain unknown. However, recent studies have indicated that genetic sex can indeed influence synaptic connectivity among shared neurons in the tail (Oren-Suissa et al. 2016) (see below), suggesting that a similar phenomenon may be at work in the C. elegans head.
Other studies of C. elegans sex pheromones have focused on a class of compounds called ascarosides. These molecules, derivatives of the dideoxy sugar ascarylose, are thought to act combinatorially to allow animals to communicate information about sex, population density, nutritional availability and more (Ludewig and Schroeder 2013). Among the ascarosides, several are produced preferentially by hermaphrodites and act as male-specific attractants. In particular, ascr#2 (also called “C6”), ascr#3 (“C9”), and ascr#8 attract males and do so synergistically when present as a mixture (Pungaliya et al. 2009; Srinivasan et al. 2008). Interestingly, another ascaroside, ascr#10, is produced preferentially by males and can specifically attract hermaphrodites, though little is known about this signal (Izrayelit et al. 2012). Furthermore, at high concentrations, ascr#3 functions as a crowding signal, triggering the dispersal of hermaphrodites but not of males (Jang et al. 2012).
In addition to a role for the male-specific CEM neurons in determining the sex-specificity of ascaroside attraction, shared neurons are also important for these responses. In particular, the ASK chemosensory neuron contributes to male ascr#3 attraction (Srinivasan et al. 2008), and the ADL chemosensory neuron is important for repressing repulsion in males (Jang et al. 2012). In neither of these cases is it known whether the genetic sex of these cells confers sex-specific functions, or instead whether they have common roles in both sexes and other alterations in circuit structure and function are responsible for this sexual dimorphism in behavior. However, recent work from our laboratory has found that the shared neuron ADF also plays a central role in male-specific pheromone attraction. The sexual state of this cell, previously implicated in dauer formation (Bargmann and Horvitz 1991) but not in sex pheromone detection, can determine an animal’s behavioral response to an ascaroside mixture. Moreover, ADF is activated by ascarosides only in males, but cell-specific masculinization enables responses in hermaphrodites (Fagan et al., in preparation). While the mechanism whereby TRA-1A modulates ADF’s sensory function is unknown, this result indicates that a key function of genetic sex in the nervous system is to implement adaptive variation in sensory repertoire.
LEARNING AND MEMORY: SEXUAL CONDITIONING
In addition to the ability of males to use secreted cues to locate hermaphrodites, the presence of hermaphrodites can modulate male learning and memory. While well-fed adults of both sexes are attracted to sodium chloride, animals can be conditioned to avoid this stimulus after pairing it with food deprivation (Saeki et al. 2001). However, adult males only display this aversive conditioning if hermaphrodites are absent during the conditioning period. When hermaphrodites are present during starvation conditioning, males override this learned NaCl aversion, a phenomenon called “sexual conditioning” (Sakai et al. 2013). This integration of starvation, hermaphrodite cues, and NaCl depends on pheromonal signals from the hermaphrodite, as well as a second, unidentified signal that is likely to be contact-dependent. Moreover, genetic feminization of the male nervous system by pan-neural expression of tra-1(gf) is sufficient to block sexual conditioning (Sakai et al. 2013). Specific feminization of the NaCl-sending ASE neuron pair is insufficient to recapitulate this effect, indicating that ASE is unlikely to have male-specific properties necessary for sexual conditioning. Nevertheless, these studies indicate that genetic sex can influence the plasticity of the nervous system as well as its innate functions.
Recently, Sammut et al. have identified a key male-specific component important for sexual conditioning (Sammut et al. 2015). In the course of other studies, these investigators discovered two interneurons in the male head, the MCMs, that had never before been detected, despite 40 years of intensive research into the C. elegans nervous system. Ablation of the MCMs causes no defects in male copulatory or food-leaving behavior, but it abolishes sexual conditioning. Remarkably, the MCMs are born during larval development through the division of a pair of differentiated glial cells (the AMso cells), a mitotic event that occurs only in males. Moreover, genetic sex-reversal of AMso is sufficient to trigger this division in hermaphrodites and to abolish it in males (Sammut et al. 2015). Thus TRA-1A regulates the ability of this glial cell to function as a neural precursor, thereby controlling the generation of a neuron necessary for sex differences in circuit plasticity.
MOTOR BEHAVIOR: DISTRIBUTED CONTROL
C. elegans navigates its environment by propagating a sinusoidal wave along its body, propelling it in the forward or reverse direction. However, the features of this behavior differ markedly between the sexes: most prominently, males bend their bodies with a higher amplitude and execute body bends more frequently (Mowrey et al. 2014). The net result of these alterations is a faster rate of locomotion and a predicted increase in the generation of forward thrust, potentially allowing the male to travel more effectively through challenging environments. In both sexes, this pattern is generated by an array of ventral cord motor neurons along the length of the animal. Interconnected sets of excitatory (cholinergic) and inhibitory (GABAergic) motor neurons allow the simultaneous contraction of muscle on one side of the body and the relaxation on the other (Zhen and Samuel 2015).
Most of the motor system is anatomically equivalent between the sexes. (Exceptions to this are the male-specific CA and CP ventral cord neurons, which are thought to be important for navigation during male copulatory behavior (Schindelman et al. 2006), as well as the hermaphrodite VC neurons, which innervate ventral body wall muscles and inhibitory motorneurons as well as the vulval muscles that control egg-laying (Schafer 2005).) This raises the possibility that modulation of the motor system driven by the genetic sex may be important for fine-tuning motor patterns. Consistent with this, sex reversal of multiple components of this system, including excitatory motor neurons and body wall muscle, alters specific features of motor behavior (Mowrey et al. 2014). Interestingly, cell-type-specific manipulations can have partial and sometimes paradoxical effects on these features, indicating that multilevel, distributed sexual modifications work together to effect optimal sex-specific locomotion. Surprisingly, a key contributor to the rate of body bends lies outside the motor system altogether: sex reversal of all worm sensory neurons significantly reduces male body-bend frequency and increases it in hermaphrodites (Mowrey et al. 2014). While the specific mechanisms underlying this effect remains unknown, it again points to an important role for genetic sex in the functional modulation of shared sensory neurons.
SEX-SPECIFIC BEHAVIOR: INTEGRATING SEX-SPECIFIC AND SHARED CIRCUITS
The most conspicuous examples of sex differences in behavior are the sex-specific behaviors of hermaphrodite egg-laying and male mating. Each of these relies heavily on sex-specific neurons. In the hermaphrodite, the HSN and VC motor neurons innervate vulval muscles to facilitate egg-laying. In the male, numerous sex-specific sensory, motor, and interneurons facilitate the stepwise behavioral program of male mating. Of course, neither of these systems operates in isolation; rather, they are deeply connected into shared circuits at all levels. While the roles of many sex-specific components in these behaviors are understood, less is known about the role of shared neurons in these sex-specific programs. However, several recent results indicate that sex-specific tuning of shared neurons may play an important role in facilitating egg-laying and male mating.
In hermaphrodites, egg-laying behavior is modulated by environment, such that unfavorable conditions lead to egg retention. Interestingly, pan-neural masculinization, or masculinization only of sensory neurons, also leads to egg retention (Lee and Portman 2007; White et al. 2007). Although the basis for this effect is not known, this observation suggests that sensory signals transduced by shared circuits might be specialized by sex, perhaps allowing hermaphrodites and males to use different criteria to assess environmental conditions. The differential sensitivity of the sexes to food-derived cues (Ryan et al. 2014) supports this notion.
In males, copulatory behavior relies heavily on the regulation of locomotion. When a male first contacts a hermaphrodite, signaling through sex-specific tail sensilla triggers a postural change (curvature of the tail) and the initiation of reverse movement. Additional tail sensilla then allow the male to locate the hermaphrodite vulval opening, upon which the male inserts its spicules and transfers sperm into the uterus (Barr and Garcia 2006). Especially during the initial steps of this process, sex-specific signals must be integrated into shared circuitry to arrest forward movement and begin reversal. The innervation of shared neurons, particularly pre-motor “command” interneurons, is necessary for this step (Sherlekar et al. 2013). Shared neurons also play a role in establishing the curved tail posture that is necessary for the male to maintain contact with its mate (Koo et al. 2011). Similarly, non-sex-specific mechanosensory neurons play an important role in the vulval search process (Liu et al. 2007). Shared neurons also produce or receive neuromodulatory signals important for facilitating male copulatory behavior and male sexual drive (Barrios et al. 2012; Garrison et al. 2012; Jee et al. 2016). Finally, ablation experiments indicate that the shared proprioceptive neuron DVA has sexually dimorphic functions (Garrison et al. 2012). Despite these many suggestions of functional modification, whether the genetic sex of these neurons regulates their development or function is not known.
In contrast, genetic sex has been shown to play a central role in altering the morphology and function of a muscle cell that is important for male copulatory behavior. In larvae of both sexes, and in adult hermaphrodites, the anal depressor muscle is involved in defecation behavior. However, in adult males, the anatomy of this cell is modified to allow it to attach to sex muscles and promote copulation. Genetically feminizing this cell disrupts this reorganization, indicating that TRA-1A, through an unknown mechanism, specifies sexually dimorphic morphogenesis of musculature (Chen and Garcia 2015).
SEX-SPECIFIC REWIRING: SYNAPTIC PRUNING GENERATES SEXUALLY DIMORPHIC CIRCUITS
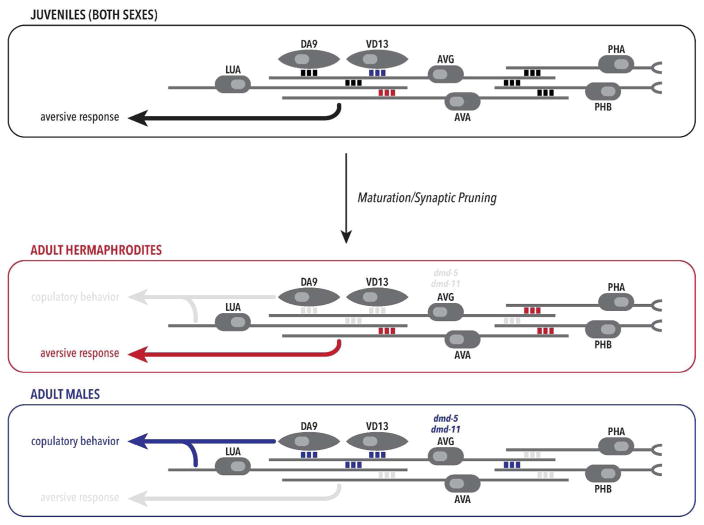
Exciting recent work has demonstrated that genetic sex can not only modulate the function of shared neurons but can also alter the pattern of connectivity among them. In the course of examining the recently described connectome of the male tail (Jarrell et al. 2012), Oren-Suissa et al. identified seven examples of apparent sexually dimorphic connectivity among cells present in both sexes (Oren-Suissa et al. 2016). Using a fluorescent labeling strategy, they found that hermaphrodites possessed three connections that were absent in adult males, while four other connections were detectible only in males (Fig. 3). In most of these cases, the processes of these neurons are similarly arranged between the sexes, indicating that synaptic specificity, not process guidance, differs by sex. These wiring patterns change the connectivity downstream of a tail chemosensory structure called the phasmid, such that signals from the phasmid in males are rerouted into circuitry important for male mating. Functional assays confirm that the male phasmid is important for efficient mating, while the hermaphrodite phasmid is involved in repulsion from aversive chemical cues (Oren-Suissa et al. 2016).
Figure 3. Genetic sex reconfigures the wiring of a shared sensory circuit.
The phasmid sensory neurons PHA and PHB, present in the tail of both sexes, receive chemical and perhaps mechanical input. Postsynaptically, sex differences in connectivity among shared neurons (indicated by triplets of red and blue boxes) act to channel this sensory input into alternative synaptic pathways (Oren-Suissa et al. 2016). In the adult hermaphrodite, signaling by PHB modulates aversive behavior. In adult male, PHB promotes copulatory behavior. Interestingly, this differential connectivity (red and blue boxes in the lower boxes) arises mostly through sex-specific pruning of synapses that initially form in embryos/larvae of both sexes (black boxes in juvenile diagram). The action of the DM genes dmd-5 and dmd-11 in the male AVG helps determine the sex-specificity of the pruning process. In contrast, other synapses (red and blue boxes in upper box) already appear to be sexually dimorphic in larvae. In all diagrams, anterior is to the right and posterior to the left.
Interestingly, for five of these sex-specific connections, sex-specific synaptogenesis is not the mechanism that generates sex differences. Rather, these connections are initially formed in larvae of both sexes. Sexual dimorphism then arises through the sex-selective pruning of these shared synapses (Oren-Suissa et al. 2016). Thus, the phasmid circuit adopts a bipotential state in larvae, and connectivity is refined as animals reach sexual maturity. The contribution of the genetic sex of specific neurons to this synaptic refinement has been investigated in one pair of sexually dimorphic connections, the hermaphrodite-specific PHB>AVA synapse and the male-specific PHB>AVG. These two connections share a presynaptic cell, PHB, whose sex is essential for the typical pattern of pruning. When PHB is masculinized in a hermaphrodite, its connection to AVA is lost, while the connection to AVG is maintained. PHB feminization in a male has the opposite effect. The genetic sex of the postsynaptic neurons is also important, though genetic sex-reversal of these cells has a milder effect on synaptic pruning. Interestingly, some of these functions of TRA-1A are non-cell-autonomous: for example, the feminization of the AVG neuron in a male causes the PHB>AVA connection to be maintained, suggesting a complex relationship between genetic sex and the control of synaptic pruning (Oren-Suissa et al. 2016).
Beyond establishing a role for TRA-1A in the control of circuit connectivity, the recent work of Oren et al. has also provided insight into how genetic sex mediates this process. Taking a candidate approach, these investigators found that mutations in two transcription factors, DMD-5 and DMD-11, disrupted sex-specific patterns of pruning (Oren-Suissa et al. 2016). These proteins belong to a conserved family of transcription factors, the DMRT (doublesex/mab-3–related transcript) or DM-domain proteins described above, that have been implicated in sex determination and sexual differentiation across the animal kingdom (Matson and Zarkower 2012). Although TRA-1A itself, like many components of sex determination pathways, does not have a conserved role outside of its own phylum, DM genes are important for sexual development in many animals, include nematodes, insects, crustaceans, birds, fish, and mammals (Matson and Zarkower 2012). In C. elegans, three DM genes (mab-3, mab-23, and dmd-3) are important direct or indirect targets of TRA-1A (Berkseth et al. 2013; Lints and Emmons 2002; Mason et al. 2008; Yi et al. 2000). However, whether DM genes also act to mediate the functions of TRA-1A in shared neurons and circuits has been unclear. The recent work of Oren-Suissa et al. indicates that DMD-5 and DMD-11 are expressed in the AVG neuron only in males, where they seem to mediate at least some of TRA-1A’s effects (Oren-Suissa et al. 2016). Whether these genes are direct TRA-1A targets in AVG remains unknown, as does the mechanism whereby these factors regulate synaptic pruning. However, the identification of a function for DM genes in sex-specific synaptic pruning in C. elegans further supports the idea that genes of this family are important modulators of sex differences in animal nervous systems.
CURRENT ISSUES AND BROADER IMPLICATIONS
Many important questions remain open with regard to the sexual modulation of shared components of the C. elegans nervous system. First, the full extent of TRA-1A function in non-sex-specific cells and circuits is unknown. Are only a few shared neurons (e.g., AWA, ADF, and AVG) subject to regulation by genetic sex, or does TRA-1A have widespread functions throughout the nervous system? Progress here will come from further functional studies of shared circuits, continued use of the genetic sex-reversal approach, and from deep sequencing to identify sex differences in gene expression in the nervous system.
Another issue is the question of TRA-1A targets. How many targets TRA-1A has in the shared nervous system, whether some are members of the DM-domain family, and how they control sexual differentiation, remain unknown. Important recent work has used a ChIP-seq approach to identify several new direct TRA-1A targets, as well numerous additional candidate targets (Berkseth et al. 2013). However, as many of TRA-1A’s regulatory functions may be highly stage-and cell-type-specific, a higher degree of resolution may be necessary for this biochemical approach be comprehensive.
The developmental regulation of the effects of genetic sex also remains a key open issue. By its nature, genetic sex is a static signal, but its effects can be manifest at highly specific time points. For example, repression of odr-10 expression in the male AWA neurons depends on the genetic sex of these cells, but this regulation seems not to occur until the larval-to-adult transition. This suggests that a temporal signal must impinge on this mechanism, perhaps to “gate” the effects of genetic sex on its regulatory targets. One possibility is that this temporal cue is communicated in the form of signals from the gonad or elsewhere. Consistent with this idea, the hormone receptor DAF-12 is important for adult male food-leaving behavior (Kleemann et al. 2008; Lipton et al. 2004), and the signals from the gonad have recently been implicated in the developmental maturation of hermaphrodite olfactory behavior (Fujiwara et al. 2016). The heterochronic pathway, a conserved genetic mechanism important for specifying developmental stage, could also play this role, acting either cell-autonomously or via a secreted signal. This possibility is supported by the finding that some heterochronic mutants, such as lep-2, disrupt sex-specific adult behaviors (Herrera et al. 2016). Understanding where and how such timing mechanisms interface with sex determination will be an important problem for the future, and raises the interesting possibility that genetic sex in C. elegans may be encoded dynamically, rather than statically, as has been seen in Drosophila (Robinett et al. 2010).
With regard to more complex systems, one important idea emerging from studies of C. elegans is that genetic sex may be thought of as a neuromodulatory cue. Neuromodulators such as neuropeptides and monoamines have profound roles in reconfiguring the functional properties of neural circuits (Marder 2012); perhaps cell-autonomous modulation of neurophysiology by genetic sex plays a similar role. Indeed, it seems likely that these two possibilities may be connected, with genetic sex influencing the production or response to such modulatory influences.
More generally, the mechanisms through which genetic sex acts in the worm nervous system may also provide insight into higher systems. Although very little is known about how non-gonadal genetic sex influences the development and function of the mammalian nervous system, multiple studies have clearly shown that it has an important role (McCarthy and Arnold 2011). Emphasis in understanding this problem has focused on the identification of functional roles for Y-linked genes (Dewing et al. 2006) or X-linked genes that escape dosage compensation in the nervous system (Berletch et al. 2015; Wu et al. 2014; Xu et al. 2008a; Xu et al. 2008b). While such factors might have important roles, it is important to keep in mind that such genetic cues in model systems—though their effects are pervasive—are very indirect. In C. elegans, early-acting dosage-sensitive genes on the X chromosome and autosome are used to read the sex-chromosome-to-autosome ratio by converging on the regulation of the autosomal gene xol-1. Once the state of xol-1 expression has been established, dosage compensation and sex determination mechanisms are implemented in parallel, and the state of the sex determination mechanism is locked into place, becoming independent of its initial chromosomal cues (Fig. 1b). Exactly how this state becomes stably established is not clear, but feedback loops are likely to be important (Berkseth et al. 2013; Hargitai et al. 2009) and epigenetic mechanisms like chromatin modification at autosomal loci, already implicated in mammals (McCarthy and Nugent 2015), could also contribute. Thus, X-and Y-linked genes in mammals could also act quite indirectly, long before the processes regulated by genetic sex takes place. Further studies into sex-biased patterns of gene expression at different developmental stages are likely to help evaluate whether such a scenario could be applicable for mammals.
Regardless, studies over the past decade have indicated that C. elegans genetic sex has a deep influence on the function of shared neurons and circuits. Sex differences in behavior clearly do not derive only from sex-specific components of the nervous system, and shared circuits, particularly in sensory systems, seem to be extensively modulated to specify sex-specific and sexually regulated behaviors. As the nervous systems of nearly all animals are much more highly isomorphic than dimorphic, modulation of these shared components seems likely to have important roles in the generation of sex-typical variation in nervous system function.
SIGNIFICANCE STATEMENT.
Simple model systems afford the opportunity to understand in detail the means by which sex influences the development, structure, and function of the nervous system. This regulation can impact behavior and give rise to sex differences in health and disease. However, little is known about how the chromosomal (“genetic”) sex of the nervous system itself contributes to these processes. In the model organism Caenorhabditis elegans, genetic sex acts in specific neurons to alter their development and modulate their function. This review discusses progress in understanding the mechanisms underlying these effects and their implications for neural circuit function and behavior.
Acknowledgments
Grant support:
Work in the author’s laboratory is supported by NIH R01 GM108885 and NSF IOS 1353075.
I thank the members of my laboratory for constructive and critical discussion, and anonymous reviewers for feedback that improved this review.
Footnotes
ROLE OF AUTHORS
D.S.P. outlined, wrote, and edited this review.
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
References
- Ankeny R. The natural history of Caenorhabditis elegans research. Nature reviews Genetics. 2001;2(6):474–479. doi: 10.1038/35076538. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251(4998):1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Barr MM, Garcia LR. Male mating behavior. WormBook: the online review of C elegans biology. 2006:1–11. doi: 10.1895/wormbook.1.78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios A, Ghosh R, Fang C, Emmons SW, Barr MM. PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nature neuroscience. 2012;15(12):1675–1682. doi: 10.1038/nn.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios A, Nurrish S, Emmons SW. Sensory regulation of C. elegans male mate-searching behavior. Current biology: CB. 2008;18(23):1865–1871. doi: 10.1016/j.cub.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkseth M, Ikegami K, Arur S, Lieb JD, Zarkower D. TRA-1 ChIP-seq reveals regulators of sexual differentiation and multilevel feedback in nematode sex determination. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):16033–16038. doi: 10.1073/pnas.1312087110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Ma W, Yang F, Shendure J, Noble WS, Disteche CM, Deng X. Escape from X inactivation varies in mouse tissues. PLoS genetics. 2015;11(3):e1005079. doi: 10.1371/journal.pgen.1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Garcia LR. Developmental alterations of the C. elegans male anal depressor morphology and function require sex-specific cell autonomous and cell non-autonomous interactions. Developmental biology. 2015;398(1):24–43. doi: 10.1016/j.ydbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell. 1999;98(3):317–327. doi: 10.1016/s0092-8674(00)81961-3. [DOI] [PubMed] [Google Scholar]
- de Bono M, Zarkower D, Hodgkin J. Dominant feminizing mutations implicate protein-protein interactions as the main mode of regulation of the nematode sex-determining gene tra-1. Genes Dev. 1995;9(2):155–167. doi: 10.1101/gad.9.2.155. [DOI] [PubMed] [Google Scholar]
- Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct Regulation of Adult Brain Function by the Male-Specific Factor SRY. Current biology: CB. 2006;16(4):415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Emmons SW. The development of sexual dimorphism: studies of the Caenorhabditis elegans male. Wiley Interdiscip Rev Dev Biol. 2014;3(4):239–262. doi: 10.1002/wdev.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan KA, Portman DS. Sexual modulation of neural circuits and behavior in Caenorhabditis elegans. Seminars in cell & developmental biology. 2014;33C:3–9. doi: 10.1016/j.semcdb.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Aoyama I, Hino T, Teramoto T, Ishihara T. Gonadal Maturation Changes Chemotaxis Behavior and Neural Processing in the Olfactory Circuit of Caenorhabditis elegans. Current biology: CB. 2016;26(12):1522–1531. doi: 10.1016/j.cub.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338(6106):540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden JM, Farboud B, Meyer BJ. Revisiting the X:A signal that specifies Caenorhabditis elegans sexual fate. Genetics. 2007;177(3):1639–1654. doi: 10.1534/genetics.107.078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargitai B, Kutnyanszky V, Blauwkamp TA, Stetak A, Csankovszki G, Takacs-Vellai K, Vellai T. xol-1, the master sex-switch gene in C. elegans, is a transcriptional target of the terminal sex-determining factor TRA-1. Development. 2009;136(23):3881–3887. doi: 10.1242/dev.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera RA, Kiontke K, Fitch DH. Makorin ortholog LEP-2 regulates LIN-28 stability to promote the juvenile-to-adult transition in Caenorhabditis elegans. Development. 2016;143(5):799–809. doi: 10.1242/dev.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes Dev. 1987;1(7):731–745. doi: 10.1101/gad.1.7.731. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination and the generation of sexually dimorphic nervous systems. Neuron S. 1991;6(2):177–185. doi: 10.1016/0896-6273(91)90354-3. [DOI] [PubMed] [Google Scholar]
- Hodgkin JA. Genetic and Anatomical Aspects of the Caenorhabditis elegans. Male. Cambridge, England: University of Cambridge; 1974. [Google Scholar]
- Hunter CP, Wood WB. The tra-1 gene determines sexual phenotype cell-autonomously in C. elegans. Cell. 1990;63(6):1193–1204. doi: 10.1016/0092-8674(90)90415-b. [DOI] [PubMed] [Google Scholar]
- Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, von Reuss SH, Genoff MC, Sternberg PW, Schroeder FC. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS chemical biology. 2012;7(8):1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012;75(4):585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, Emmons SW. The connectome of a decision-making neural network. Science. 2012;337(6093):437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- Jee C, Goncalves JF, LeBoeuf B, Garcia LR. CRF-like receptor SEB-3 in sex-common interneurons potentiates stress handling and reproductive drive in C. elegans. Nat Commun. 2016;7:11957. doi: 10.1038/ncomms11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann G, Jia L, Emmons SW. Regulation of Caenorhabditis elegans male mate searching behavior by the nuclear receptor DAF-12. Genetics. 2008;180(4):2111–2122. doi: 10.1534/genetics.108.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo PK, Bian X, Sherlekar AL, Bunkers MR, Lints R. The robustness of Caenorhabditis elegans male mating behavior depends on the distributed properties of ray sensory neurons and their output through core and male-specific targets. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(20):7497–7510. doi: 10.1523/JNEUROSCI.6153-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Portman D. Neural sex modifies the function of a C. elegans sensory circuit. Current biology: CB. 2007;17(21):1858–1863. doi: 10.1016/j.cub.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Leighton DH, Choe A, Wu SY, Sternberg PW. Communication between oocytes and somatic cells regulates volatile pheromone production in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(50):17905–17910. doi: 10.1073/pnas.1420439111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton DH, Sternberg PW. Mating pheromones of Nematoda: olfactory signaling with physiological consequences. Curr Opin Neurobiol. 2016;38:119–124. doi: 10.1016/j.conb.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Lints R, Emmons SW. Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 2002;16(18):2390–2402. doi: 10.1101/gad.1012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(34):7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kim K, Li C, Barr MM. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(27):7174–7182. doi: 10.1523/JNEUROSCI.1405-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig AH, Schroeder FC. Ascaroside signaling in C. elegans. WormBook: the online review of C elegans biology. 2013:1–22. doi: 10.1895/wormbook.1.155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76(1):1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D, Rabinowitz J, Portman D. dmd-3, a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development. 2008;135(14):2373–2382. doi: 10.1242/dev.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nature reviews Genetics. 2012;13(3):163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. At the frontier of epigenetics of brain sex differences. Front Behav Neurosci. 2015;9:221. doi: 10.3389/fnbeh.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrey WR, Bennett JR, Portman DS. Distributed Effects of Biological Sex Define Sex-Typical Motor Behavior in Caenorhabditis elegans. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(5):1579–1591. doi: 10.1523/JNEUROSCI.4352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan A, Venkatachalam V, Durak O, Reilly DK, Bose N, Schroeder FC, Samuel AD, Srinivasan J, Sternberg PW. Contrasting responses within a single neuron class enable sex-specific attraction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(10):E1392–1401. doi: 10.1073/pnas.1600786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Suissa M, Bayer EA, Hobert O. Sex-specific pruning of neuronal synapses in Caenorhabditis elegans. Nature. 2016;533(7602):206–211. doi: 10.1038/nature17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, White JG, LeBoeuf B, Garcia LR, Alon U, Hobert O. A cellular and regulatory map of the cholinergic nervous system of C. elegans. Elife. 2015:4. doi: 10.7554/eLife.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman D. Genetic Control of Sex Differences in C. elegans Neurobiology and Behavior. Adv Genet. 2007;59:1–37. doi: 10.1016/S0065-2660(07)59001-2. [DOI] [PubMed] [Google Scholar]
- Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Miller L, Kopczynski J, Meyer B. xol-1 acts as an early switch in the C. elegans male/hermaphrodite decision. Cell S. 1995;80(1):71–82. doi: 10.1016/0092-8674(95)90452-2. [DOI] [PubMed] [Google Scholar]
- Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS biology. 2010;8(5):e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DA, Miller RM, Lee K, Neal SJ, Fagan KA, Sengupta P, Portman DS. Sex, age, and hunger regulate behavioral prioritization through dynamic modulation of chemoreceptor expression. Current biology: CB. 2014;24(21):2509–2517. doi: 10.1016/j.cub.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. The Journal of experimental biology. 2001;204(Pt 10):1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- Sakai N, Iwata R, Yokoi S, Butcher RA, Clardy J, Tomioka M, Iino Y. A sexually conditioned switch of chemosensory behavior in C. elegans. PloS one. 2013;8(7):e68676. doi: 10.1371/journal.pone.0068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut M, Cook SJ, Nguyen KC, Felton T, Hall DH, Emmons SW, Poole RJ, Barrios A. Glia-derived neurons are required for sex-specific learning in C. elegans. Nature. 2015;526(7573):385–390. doi: 10.1038/nature15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer WR. Egg-laying. WormBook: the online review of C elegans biology. 2005:1–7. doi: 10.1895/wormbook.1.38.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman G, Whittaker A, Thum J, Gharib S, Sternberg P. Initiation of male sperm-transfer behavior in Caenorhabditis elegans requires input from the ventral nerve cord. BMC biology. 2006;4:26. doi: 10.1186/1741-7007-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84(6):899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Sherlekar AL, Janssen A, Siehr MS, Koo PK, Caflisch L, Boggess M, Lints R. The C. elegans male exercises directional control during mating through cholinergic regulation of sex-shared command interneurons. PloS one. 2013;8(4):e60597. doi: 10.1371/journal.pone.0060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn H, Teal P, Malik R, Edison A, Sternberg P, Schroeder F. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454(7208):1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Developmental biology. 1980;78(2):542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Postembryonic cell lineages of the nematode Caenorhabditis elegans. Developmental biology. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Developmental biology. 1983;78:542–576. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83(2):207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- White J, Nicholas T, Gritton J, Truong L, Davidson E, Jorgensen E. The sensory circuitry for sexual attraction in C. elegans males. Current biology: CB. 2007;17(21):1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- White JQ, Jorgensen EM. Sensation in a single neuron pair represses male behavior in hermaphrodites. Neuron. 2012;75(4):593–600. doi: 10.1016/j.neuron.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AS. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. BioEssays: news and reviews in molecular, cellular and developmental biology. 1995;17(1):71–77. doi: 10.1002/bies.950170113. [DOI] [PubMed] [Google Scholar]
- Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nature reviews Genetics. 2009;10(11):797–804. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- Wolff JR, Zarkower D. Somatic sexual differentiation in Caenorhabditis elegans. Current topics in developmental biology. 2008;83:1–39. doi: 10.1016/S0070-2153(08)00401-8. [DOI] [PubMed] [Google Scholar]
- Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, Smallwood PM, Erlanger B, Wheelan SJ, Nathans J. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron. 2014;81(1):103–119. doi: 10.1016/j.neuron.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PloS one. 2008a;3(7):e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008b;28(17):4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Ross JM, Zarkower D. mab-3 is a direct tra-1 target gene regulating diverse aspects of C. elegans male sexual development and behavior. Development. 2000;127(20):4469–4480. doi: 10.1242/dev.127.20.4469. [DOI] [PubMed] [Google Scholar]
- Zarkower D, Hodgkin J. Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell. 1992;70(2):237–249. doi: 10.1016/0092-8674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Zhen M, Samuel AD. C. elegans locomotion: small circuits, complex functions. Curr Opin Neurobiol. 2015;33:117–126. doi: 10.1016/j.conb.2015.03.009. [DOI] [PubMed] [Google Scholar]