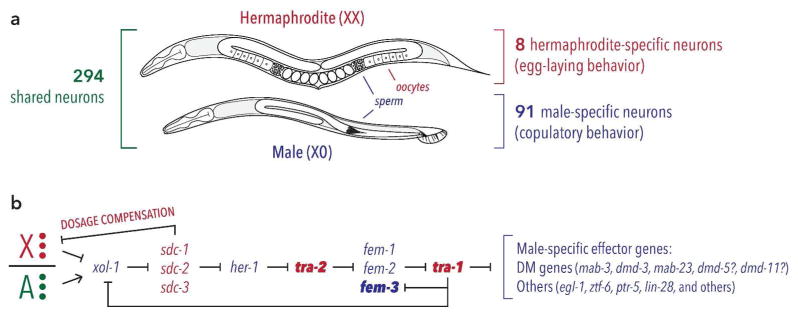
Figure 1. Sexual dimorphism and sex determination in the nematode C. elegans.
(A) Adult males and hermaphrodites share an overall body plan but differ in germline, gonad, genitalia, and many other features. Adults of both sexes possess exactly 294 shared neurons, while adult hermaphrodites and adult males possess 8 and 91 sex-specific neurons, respectively. (B) The genetic hierarchy controlling C. elegans sex determination in the soma is triggered by the ratio of sex chromosomes to autosomes (X/A ratio). Genes active in XX animals are shown in red, while those active in XO animals are shown in blue. The X/A ratio is “calculated” through the molecular activities of dosage-sensitive X- and A-linked genes (red and green circles, respectively), which converge on the regulation of the autosomal gene xol-1. As a result of this regulation, xol-1 is expressed only in XO embryos, where it regulates both sexual differentiation and well as dosage compensation by repressing the sdc genes. Downstream of the sdc genes, dosage compensation and sexual differentiation are implemented through independent pathways. At the terminus of the sex determination pathway lies the “master regulator” tra-1, shown in bold. Acting via its product TRA-1A, this gene is both necessary and sufficient for essentially all sex differences in the soma, including the nervous system. Several known direct targets of TRA-1A are shown, with question marks indicating targets that have been suggested to be direct. TRA-1A also regulates xol-1 and fem-3, providing feedback that likely stabilizes the state of the pathway once dosage compensation equalizes the X-to-A ratio. tra-2 and fem-3, both shown in bold, are normally active in only in hermaphrodites or males, respectively. Forced expression of these genes in specific tissues of the opposite sex is largely sufficient to reverse their sexual state by activating or inhibiting TRA-1A.