Abstract
Ischemic stroke is a leading cause of death and disability in the United States. It is known that males and females respond differently to stroke. Depending on age, the incidence, prevalence, mortality rate, and disability outcome of stroke differ between the sexes. Females generally have strokes at older ages than males and, therefore, have a worse stroke outcome. There are also major differences in how the sexes respond to stroke at the cellular level. Immune response is a critical factor in determining the progress of neurodegeneration after stroke and is fundamentally different for males and females. Additionally, females respond to stroke therapies differently from males, yet they are often left out of the basic research that is focused on developing those therapies. With a resounding failure to translate stroke therapies from the bench to the bedside, it is clearer than ever that inclusion of both sexes in stroke studies is essential for future clinical success. This Mini-Review examines sex differences in the immune response to experimental stroke and its implications for therapy development.
Keywords: experimental stroke, sex difference, immune response, neuroinflammation, infarct volume, therapy
Stroke is a leading cause of death and disability, affecting over 7 million people in the United States (Towfighi and Saver, 2011; Onwuekwe and Ezeala-Adikaibe, 2012; Mozaffarian et al., 2015). Ischemic stroke is caused by the occlusion of a blood vessel in the brain and constitutes the majority of all strokes. It has been widely reported that, at younger ages, men exhibit an increase in stroke incidence compared with women (Reeves et al., 2008). However, this discrepancy disappears with advancing age, causing stroke incidence in women to become equal if not greater than that in men, depending on the study (Kissela et al., 2004; Rothwell et al., 2005; Lofmark and Hammarstrom, 2007). Between the ages of 45 and 74 years, women also have significantly lower risk of stroke mortality than men (Reeves et al., 2008). Similarly to stroke incidence, the mortality benefit for women is lost with older age, and women over the age of 85 years actually have higher stroke mortality than men (Reeves et al., 2008). Stroke prevalence is about the same for the sexes; however, because there are more females of older age, there are substantially more female stroke survivors than male stroke survivors (Neyer et al., 2007). In fact, over the age of 85 years, there are two- to threefold more women with stroke, depending on race (Howard et al., 2005). Women also have poorer stroke outcomes than men and are more likely to suffer from disability, depression, and a lower quality of life (Di Carlo et al., 2003). The discrepancy in quality of life for women after stroke may be attributed, in part, to older age at stroke onset, poorer baseline health prior to stroke, less social support after stroke, greater chance of being widowed prior to stroke, and a lower chance of receiving stroke therapies at diagnosis (Reeves et al., 2008).
SIGNIFICANCE.
Males and females respond differently to stroke, and those differences are largely the result of cellular and molecular sex hormone-independent mechanisms. This Mini-Review focuses on sex differences in the immune response and inflammation to mouse models of ischemic stroke. We additionally address how the failure to examine female-specific responses to stroke and test therapies in female stroke models has, in part, contributed to the overall lack of clinical stroke therapeutic success. These findings support the requirement for representation of females in basic stroke therapy research.
BASIC STUDIES IN ANIMAL MODELS OF STROKE
There are many methods for mimicking ischemic stroke in rodents. Each model has advantages and disadvantages in terms of its ability to replicate human ischemic events and reproducibility (Casals et al., 2011; Canazza et al., 2014; Fluri et al., 2015). The permanent or transient middle cerebral artery occlusion (MCAO) model is one of the best-characterized models of ischemic stroke because of its reproducibility and its ability to simulate human stroke closely (Fluri et al., 2015). Multiple studies using in vivo rodent models of stroke have demonstrated that young females have smaller infarcts than males after ischemic injury (Alkayed et al., 1998; Murphy et al., 2004; Lang and McCullough, 2008; Vagnerova et al., 2008; Cheng and Hurn, 2010; Siegel et al., 2010; Banerjee et al., 2013; Manwani et al., 2013; Dotson et al., 2015). However, just as in humans, in whom ischemic protection is decreased in postmenopausal women, aging female rodents (14–22 months) no longer display a protective phenotype to experimental stroke and can have even larger infarcts compared with males of the same age (Manwani et al., 2013). Rodent stroke models can be extremely useful because of their ability to identify and isolate specific ischemic mechanisms. The roles of sex hormones, inflammation, and the immune response to stroke have all been detailed in rodent stroke models, although more so in young male rodents.
SEX HORMONES IN ANIMAL STROKE MODELS
The effect of sex hormones on stroke can be observed in rodent disease models. As discussed above, protection against ischemic brain injury after experimental stroke in young adult female rats disappears after reproductive senescence (Alkayed et al., 2000). The same study found that ovarian hormones restore ischemic protection in reproductive, senescent female rats, independent of blood flow. Alternatively, blood flow mechanism is indicative of hormone protection in young female rats compared with male or ovariectomized female rats after experimental stroke (Alkayed et al., 1998). Sex steroids also modulate STAT3, a transcription factor that is activated in neurons after cerebral ischemia in a sex-specific manner, with higher activation in females than in males (Di Domenico et al., 2012). In humans, however, treatment of stroke in postmenopausal women with exogenous estrogen has failed to show any benefit and has even shown increased risk of stroke compared with placebo treatment (Simon et al., 2001; Viscoli et al., 2001; Hendrix et al., 2006). Despite the mechanism of sex hormone protection during stroke, the failure of human estrogen replacement clinical trials indicates a cellular and molecular, sex hormone-independent means of protection.
IMMUNE CELLS HAVE SEX DIFFERENCES
It is increasingly clear that males and females exhibit unique responses to disease and that differences in the immune system contribute greatly to those disparities. Biological sex shapes how our immune cells function in various circumstances and leads to sex-specific outcomes. For example, females initiate an elevated immune response to antigenic challenge compared with males, leading to a more efficient clearance of pathogens yet also promoting a higher incidence of inflammatory and auto-immune diseases (Klein, 2012). Among the total population diagnosed with an autoimmune disease, almost 80% are female (Libert et al., 2010). Females exhibit greater toll-like receptor expression (Klein et al., 2010) and an increase in number and function of monocytes, macrophages, and dendritic cells than males (Boissier et al., 2003; Xia et al., 2009; Melgert et al., 2010). Additionally, antigen-presenting cells from females present peptides better than the same cells from males (Weinstein et al., 1984). Females also exhibit an overall heightened adaptive immune response that specifically leads to a Th1-skewed response (Villacres et al., 2004). Sex differences in cellular immune function directly correlate with increases in diseases such as Sjogren’s syndrome, Hashimoto’s thyroiditis, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, asthma, inflammatory bowel disease, allergy, and eczema for females (Okoro and Kane, 2009; Libert et al., 2010; Ziyab et al., 2010; Tam et al., 2011).
ISCHEMIC DAMAGE AND INFLAMMATION
Brain-specific cellular and molecular responses reflect more inflammatory microglia after experimental stroke in young male mice compared with females (Banerjee et al., 2013; Dotson et al., 2015). Microglia cultured from young female MCAO mice show a reduced expression of interleukin (IL)-1β and macrophage inflammatory protein-1α compared with male MCAO mice (Bodhankar et al., 2015). Young male mice also exhibit increased expression of inflammatory genes in the ischemic brain after experimental stroke compared with females, including genes associated with T-cell function, adhesion molecules, cell signaling, major histocompatibility complex (MHC) and costimulatory signals, cell death, and inflammatory cytokines (Dotson et al., 2015). Distinct changes in miRNAs after ischemic events in male and female brains have been found to influence mechanisms underlying sexually dimorphic responses to stroke in addition to an miRNA signature response to ischemia that is common to both sexes (Lusardi et al., 2014). Even microvasculature in the brain contributes to sex differences in stroke. Endothelial cells isolated from the microvasculature of female brains are more resistant to ischemic injury compared with endothelial cells in males, which can be attributed to lower expression of soluble epoxide hydrolase and higher levels of vasoprotective epoxyeicosatrienoic acids (Gupta et al., 2012; Davis et al., 2013; Zhang et al., 2013).
PERIPHERAL IMMUNE RESPONSE TO ISCHEMIC STROKE
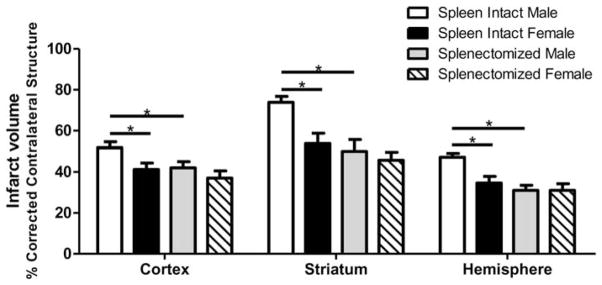
After ischemic stroke, peripheral leukocytes become activated and can migrate to the site of injury, perpetuating neurological damage (Offner et al., 2006a,b; Seifert et al., 2012a,b). It has been widely reported that the spleen, specifically, contributes to stroke outcome and, as reported in humans (Sahota et al., 2013), the spleens of rodents decrease in size after stroke (Offner et al., 2006a,b; Seifert et al., 2012a,b). Young male rodent stroke models demonstrate that ischemic injury causes the spleen to release inflammatory immune cells into the periphery and that the elimination of the spleen significantly improves stroke outcome (Ajmo et al., 2008; Ostrowski et al., 2012; Pennypacker and Offner, 2014; Dotson et al., 2015). Splenic atrophy associated with stroke is also accompanied by an increase in circulating macrophages in the blood (Offner et al., 2006b). The peripheral immune response to stroke is sex specific in both the spleen and the blood. Young male mice have a greater frequency of activated CD4+ T cells and more VLA-4-expressing cells in the spleen after experimental stroke than female mice (Banerjee et al., 2013). Moreover, splenectomy prior to MCAO significantly reduces infarct volume and activated microglia in the brain of male but not female mice, indicating that the peripheral immune response is more influential for stroke outcome in males (Dotson et al., 2015; Fig. 1). Splenectomy before MCAO also significantly reduces circulating macrophages/monocytes and activated T cells in males but not females, resulting in reduction of ischemic damage in males but not females (Dotson et al., 2015).
Fig. 1.
Splenectomy attenuates sex differences in infarct volume following experimental stroke in mice. Male and female mice were subjected to sham splenectomy or splenectomy 2 weeks prior to transient MCAO (60 min). Brains were harvested 96 hr after MCAO, and brain slices were stained with 2,3,5-triphenyltetrazolium. Infarct volumes were measured as a percentage of contralateral structure. Values are mean ±SEM of 11 spleen-intact male and female mice; n =12 for splenectomized males and females. ★P <0.05, two-way ANOVA. Reprinted from Journal of Neuroimmunology, 15;278, Dotson, et al., Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke, 289–98, 2015, with permission of Elsevier.
REGULATORY FACTORS IN ISCHEMIC STROKE
Factors that regulate the immune response have the potential to be attractive therapeutic targets for stroke. Foxp3 regulatory T cells (Tregs) are critical regulators of inflammatory disease, such as autoimmunity, and are differentially controlled between the sexes. Recent findings indicate that Foxp3 Tregs are regulated by hormonal fluctuations that can disrupt the balance between Tregs and effector immune cells, causing different sex responses to inflammation and autoimmune disease (Nie et al., 2015). In young male mice, the frequency of CD4+Foxp3+ Tregs in the spleen increases after MCAO (Offner et al., 2006b). However, the data on whether CD4+ regulatory T cells resolve or intensify stroke-related neuronal tissue damage are conflicting (Schabitz, 2013; Xu et al., 2013). Some studies have indicated that CD4+ Tregs can protect against brain tissue damage following stroke and, therefore, improve stroke outcome (Liesz et al., 2009; Planas and Chamorro, 2009; Li et al., 2013). Other studies have identified Tregs as harmful aggravators of neurodegeneration (Kleinschnitz and Wiendl, 2013; Kleinschnitz et al., 2013) or, alternatively, have indicated that CD4+ Tregs have no influence on ischemic damage at all (Ren et al., 2011; Gu et al., 2012; Stubbe et al., 2013). Additionally, there have been no observable differences in the frequency of splenic or circulating CD4+ Tregs between young male and female mice after MCAO (Dotson et al., 2015, 2016b). The existence of conflicting reports on the role of CD4+ Tregs during stroke demonstrates the requirement for more such studies in both sexes and the identification of additional immunoregulatory factors in stroke. For example, the effects of CD8+ Tregs on stroke have been examined even less than those of CD4+ Tregs. CD8+ Tregs express the cell surface molecule CD122 and can kill or suppress activated immune cells with perforin or immunosuppressive cytokines such as IL-10 (Wang and Alexander, 2009). CD8+CD122+ Tregs are directly correlated with a better initial stroke outcome and are specifically more prevalent in the ischemic tissue of young female mice after MCAO than in males (Banerjee et al., 2013; Bodhankar et al., 2013). Young female mice also have more circulating CD8+ Tregs after MCAO than males (Dotson et al., 2015). However, studies in humans have indicated that an increase in serum IL-10 in females after stroke is associated with poorer acute and long-term outcome and suggest that IL-10 levels may be a result of factors that interact with sex, such as age and stroke severity (Conway et al., 2015).
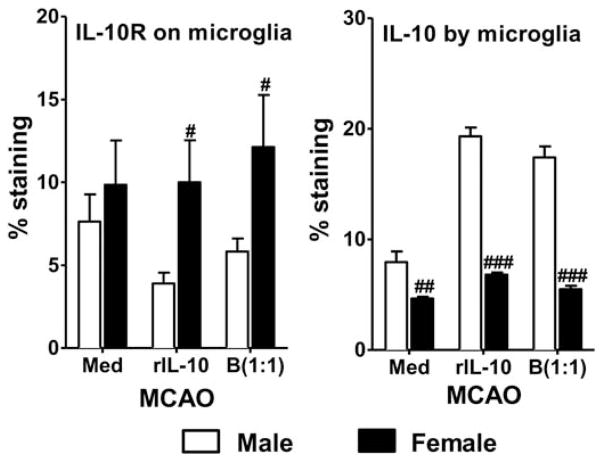
Regulatory microglia and macrophages that display an M2 phenotype have recently been implicated in neuroprotection (Chu et al., 2012; Jalal et al., 2012; Wang et al., 2013; Hu et al., 2015). Ischemic events lead to an increase in promotion of the M1 phenotype of microglia and infiltrating macrophages which, in turn, perpetuate cell death and inflammation (Schilling et al., 2005; Denker et al., 2007; Hu et al., 2012). Anti-inflammatory cytokines such as IL-10 and IL-4 can promote the M2 phenotype, thereby improving stroke outcome (Xiong et al., 2011; Hu et al., 2012; Perez-de Puig et al., 2013; Cherry et al., 2014). Exposure to recombinant IL-10 or IL-10-producing regulatory B cells dampens production of inflammatory cytokines in cultured MCAO microglia from both male and female young mice, but the reduction is significantly greater in females (Bodhankar et al., 2015). Treatment with IL-10-producing B cells also increases the expression of the M2 marker CD206 in microglia from both male and female mice (Fig. 2) while increasing IL-10 receptors on female microglia and IL-10 production by male microglia (Bodhankar et al., 2015; Fig. 3).
Fig. 2.
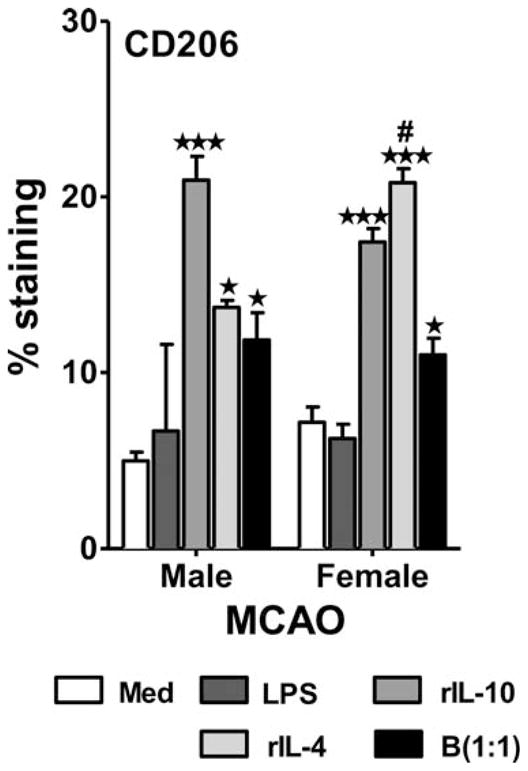
Treatment with IL-10+ B cells promotes M2 phenotype induction in microglia post-MCAO. Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluence) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10 ng/ml LPS for 4 hr. Supernatants were discarded after 4 hr, and one of the following treatments was given in 1 ml fresh culture medium: no treatment, 20 ng recombinant IL-10 (rIL-10), 20 ng/ml rIL-4, or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37 °C and 5% CO2 for 24 hr, and the M2 marker CD0206 was determined by flow cytometry. Values are mean ±SEM. Data are representative of two separate coculture setups, with each treatment condition performed in duplicate for every experimental setup. Statistical analysis was performed by one-way ANOVA, with post hoc Dunnett test. Statistical differences between the two sexes were determined by two-way ANOVA followed by post hoc Bonferroni multiple-comparisons test. ★P ≤ 0.05, ★ ★ ★P ≤0.001, significant differences between sample means compared with the LPS-stimulated condition; #P ≤0.05 compared with the corresponding treatment in male MG. Reprinted from Metabolic Brain Disease, Role for microglia in sex differences after ischemic stroke: importance of M2, 30, 2015, 1515–1529, Bodhankar et al., © Springer Science+Business Media New York 2015, With permission of Springer.
Fig. 3.
Increased microglial surface expression of IL-10R by female microglia and IL-10 production by male microglia post-MCAO. Primary MG isolated and cultured from MCAO-treated WT male and female mice were harvested after 21 days in vitro (at confluence) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10 ng/ml LPS for 4 hr. Supernatants were discarded after 4 hr, and one of the following treatments was given in 1 ml fresh culture medium: no treatment, 20 ng recombinant IL-10 (rIL-10), or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37 °C and 5% CO2 for 24 hr, and IL-10 receptor (IL-10R) or IL-10 production was determined by flow cytometry. Values are mean ±SEM. Data are representative of two separate coculture setups, with each treatment condition performed in duplicate for every experimental setup. Statistical differences between the two sexes were determined by two-way ANOVA, followed by post hoc Bonferroni multiple-comparisons test. #P ≤0.05, ##P ≤0.01, ###P ≤0.001 compared with the corresponding treatment in male MG. Reprinted from Metabolic Brain Disease, Role for microglia in sex differences after ischemic stroke: importance of M2, 30, 2015, 1515–1529, Bodhankar et al., © Springer Science+Business Media New York 2015, With permission of Springer.
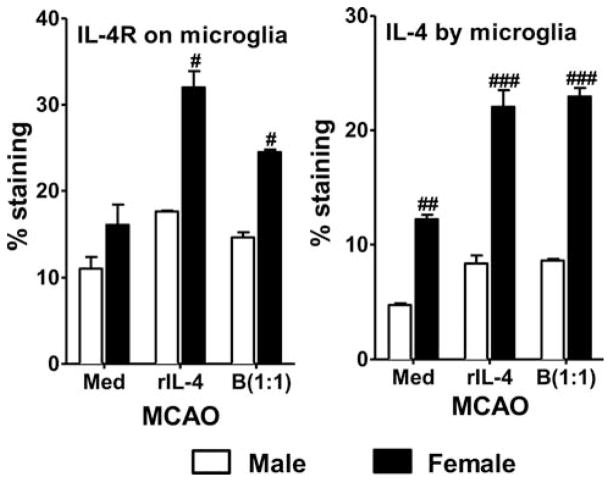
IL-4 has potent anti-inflammatory activity and plays a crucial role in stroke outcome. IL-4 differentiates antigen-stimulated naive T cells into Th2 cells that secrete anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 that, consequently, suppress Th1 inflammatory effector cells (Abbas et al., 1996). In young male mice, loss of IL-4 signaling results in an increase in infarct volume and inflammation, which is reversed with replacement of exogenous IL-4 (Xiong et al., 2011). Another study conducted with young male mice suggests that IL-4 is preferentially produced and secreted by neurons after ischemic stroke and leads to an increase of IL-4R on microglia, providing more evidence for IL-4 as an endogenous defense mechanism to stroke (Zhao et al., 2015). IL-4 has also been shown to be important for neuroprotection of young female mice after stroke. Loss of IL-4 in female mice exacerbated infarct and increased inflammatory cells in the brain, thus eliminating sex difference after experimental stroke (Xiong et al., 2015). M2 microglia are reduced in the brains of female IL-4 KO mice but not males, supporting the neuroprotective role of IL-4 in females (Xiong et al., 2015). Additionally, treatment of MCAO microglia cells with regulatory B cells preferentially increases IL-4R expression and IL-4 production by female cells (Bodhankar et al., 2015; Fig. 4).
Fig. 4.
Treatment with IL-10+ B cells leads to increased IL-4R expression and IL-4 production by female microglia post-MCAO. Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluence) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10 ng/ml LPS for 4 hr. Supernatants were discarded after 4 hr, and one of the following treatments was given in 1 ml fresh culture medium: no treatment, 20 ng recombinant IL-4 (rIL-4), or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37 °C and 5% CO2 for 24 hr and IL-4R expression and IL-4 production were determined by flow cytometry. Values are mean ±SEM. Data are representative of two separate coculture setups, with each treatment condition performed in duplicate for every experimental setup. Statistical differences between the two sexes were determined by two-way ANOVA, followed by post hoc Bonferroni multiple-comparisons test. #P ≤0.05, ##P ≤0.01, ###P ≤0.001 compared with the corresponding treatment in male MG. Reprinted from Metabolic Brain Disease, Role for microglia in sex differences after ischemic stroke: importance of M2, 30, 2015, 1515–1529, Bodhankar et al., © Springer Science+Business Media New York 2015, With permission of Springer.
TRANSLATION OF THERAPIES TO BOTH SEXES
The difference in how males and females respond to stroke must be a major consideration when designing new stroke therapies. Numerous therapies have exhibited neuroprotection in the laboratory but have failed to translate to clinical success, in part because of the lack of females in basic research (Turner et al., 2013). One promising target for stroke therapy is peroxisome proliferator-activated receptor-α (PPARα). PPARα is a nuclear hormone receptor and ligand-activated transcription factor that negatively regulates inflammation via the nuclear factor-κB signaling pathway (Delerive et al., 2001). PPARα also plays a role in oxidative stress and cell adhesion (Poynter and Daynes, 1998; Marx et al., 1999). PPARα is widely expressed in the body, but it is of particular interest as a target for stroke therapy because of its expression in brain tissue and in the white pulp of the spleen (Kainu et al., 1994; Braissant et al., 1996; Culling-ford et al., 1998; Bishop-Bailey, 2000; Moreno et al., 2004). PPARα is expressed at the cellular level in macrophages, T cells, and B cells (Chinetti et al., 1998; Jones et al., 2002).
PPARα agonist treatment has been effective at neuroprotection from stroke in male mice when given prior to stroke onset. PPARα agonist improves stroke outcome by reducing adhesion molecule expression in the brain and, thereby, limiting early neutrophil infiltration, reducing inflammatory factors, regulating oxidative stress, and increasing cerebral blood flow (Deplanque et al., 2003; Collino et al., 2006; Guo et al., 2010; Ouk et al., 2013, 2014; Losey et al., 2015). However, PPARα agonists did not have the same protective effect in female mice subjected to MCAO (Dotson et al., 2016b). The lack of therapeutic translation to females is likely due to lower expression of PPARα receptors in female tissues and immune cells and, therefore, a lack of immune regulation after stroke, specifically in the periphery (Jalouli et al., 2003; Dunn et al., 2007; Dotson et al., 2015, 2016b, 2016c).
Another potential stroke target is poly(ADP-ribose) polymerase (PARP1). Loss of PARP1 in young male mice led to neuroprotection from stroke (Eliasson et al., 1997; McCullough et al., 2005). However, young female PARP1 KO mice or WT mice treated with a PARP1 inhibitor had no protection or intensified neurological damage after ischemic injury (Hagberg et al., 2004; McCullough et al., 2005). Minocycline is a tetracycline derivative used to treat bacterial infections. Minocycline acts as a PARP inhibitor and, therefore, has neuroprotective activity in neurodegenerative disorders (Yrjanheikki et al., 1999; Alano et al., 2006; Lampl et al., 2007). When both sexes were tested in mouse models of stroke, minocycline was ineffective at reducing ischemic damage in females subjected to the MCAO model (Li and McCullough, 2009), but it did improve females’ stroke outcome in a thromboembolic model (Hoda et al., 2011).
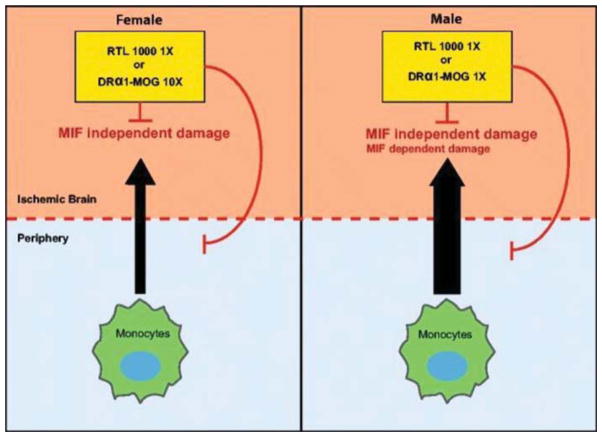
Recombinant T-cell receptor ligand (RTL) has been uysed to treat experimental stroke in mice (Burrows et al., 1998, 2001; Vandenbark et al., 2003; Huan et al., 2004; Subramanian et al., 2009; Akiyoshi et al., 2011; Dotson et al., 2014, 2016a). RTL molecules consist of the α1 and β1 domains of MHC class II molecules expressed as a single polypeptide with or without antigenic amino terminal extensions (Burrows et al., 1999; Vandenbark et al., 2003). RTLs are partial agonists that deactivate effector T cells to become nonresponsive (Burrows et al., 2001; Wang et al., 2003). RTL inhibits binding of macrophage migration inhibitory factor (MIF) to CD74 and blocks downstream inflammatory effects in the CNS by directly binding to and downregulating the cell surface expression of CD74 (MHC class II invariant chain) on CD11b+ monocytes (Benedek et al., 2013; Vandenbark et al., 2013). RTL1000 consists of a human leukocyte antigen–antigen D-related (HLA-DR) 2 moiety linked to human myelin oligodendrocyte glycoprotein (MOG)-35–55 peptide (Offner et al., 2011) and has been shown to resolve infarct in young male and female humanized DR2 mice subjected to MCAO (Akiyoshi et al., 2011; Pan et al., 2014). HLA-DRα1–MOG-35–55 is a novel recombinant protein consisting of the HLA-DRα1 domain linked to MOG-35–55 peptide but lacking the β1 domain found in RTL (Benedek et al., 2014). HLA-DRα1–MOG-35–55 has also been shown to reduce infarct volume significantly after MCAO in young male mice yet requires a tenfold greater concentration to achieve the same result in female mice (Benedek et al., 2014; Pan et al., 2014). As shown in Figure 5, sex differences in inflammation that are mediated through MIF/CD74 interactions or MIF-independent mechanisms are thought to be the reason for this discrepancy (Pan et al., 2014).
Fig. 5.
RTL1000 and DRα1–MOG-35–55 treatment of MCAO. RTL1000, which consists of the HLA-DR2β1α1 domains linked to human MOG-35–55 peptide and DRα1–MOG-35–55 that lacks the polymorphic β1 domain, can reduce infarct size in female and male mice after MCAO. In males, in which peripheral immune cells cause greater MCAO damage than in females and in which there may be some MIF-dependent inflammation, RTL1000 and DRα1–MOG-35–55 have the same treatment potency. In females, which appear to have little if any MIF-dependent MCAO damage, treatment with RTL1000 is tenfold more potent than treatment with DRα1–MOG-35–55. This differential treatment effect between males and females could be based on gender-associated effects of MIF on infarct size, recruitment of peripheral immune cells into the ischemic brain, or axonal death. Reprinted from Translational Stroke Research, Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke, 5(5), 2014, 577–585, Pan et al. Copyright © The Author(s) 2014.
CONCLUSIONS
As the U.S. population ages, the incidence of stroke will likely increase. Stroke prevalence is projected to increase by 3.4 million individuals, and medical costs associated with stroke will triple between the years 2012 and 2030 (Ovbiagele et al., 2013). This increase will be disproportionate for the eldery female population and will accentuate stroke-related disabilities (Lai et al., 2005; Manwani and McCullough, 2011). Prior preclinical trials have failed to yield reproducible results or effective new stroke therapies. Thus, standardized guidelines for study design and data reporting in preclinical randomized controlled trials are required for successful translation of promising experimental approaches to effective clinical therapies (Llovera and Liesz, 2016). Therefore, the inclusion of sex, age, multiple disease models, and comorbidities in the study design of basic research is of utmost importance.
Acknowledgments
This work was supported by NIH/NINDS 5R01NS076013 and 5R01NS075887. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Contract grant sponsor: National Institute of Neurological Disorders and Stroke; Contract grant number: 5R01NS076013; Contract grant number: 5R01NS075887; Contract grant sponsor: Department of Veterans Affairs, Veterans Health Administration Office of Research and Development, Biomedical Laboratory Research and Development.
The authors thank Gail Kent for assistance with manuscript submission. The content of this Mini-Review do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- GM-CSF
granulocyte macrophage colony stimulating factor
- LPS
lipopolysaccharide
- MCAO
middle cerebral artery occlusion
- MG
microglia
- MHC
major histocompatibility complex
- Treg
regulatory T cell
Footnotes
CONFLICT OF INTEREST STATEMENT
H. Offner and Oregon Health and Science University have significant financial interests in Artielle Immunotherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the Oregon Health and Science University and Veterans Affairs Portland Health Care System Conflict of Interest in Research Committees. H. Offner discloses U.S. Patent No. 8,491,913 B2 for the use of recombinant molecules in treatment of stroke.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi K, Dziennis S, Palmateer J, Ren X, Vandenbark AA, Offner H, Herson PS, Hurn PD. Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res. 2011;2:404–410. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Meza-Romero R, Andrew S, Leng L, Burrows GG, Bourdette D, Offner H, Bucala R, Vandenbark AA. Partial MHC class II constructs inhibit MIF/CD74 binding and downstream effects. Eur J Immunol. 2013;43:1309–1321. doi: 10.1002/eji.201243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Zhu W, Libal N, Casper A, Yu X, Meza-Romero R, Vandenbark AA, Alkayed NJ, Offner H. A novel HLA-DRalpha1-MOG-35–55 construct treats experimental stroke. Metab Brain Dis. 2014;29:37–45. doi: 10.1007/s11011-013-9440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000;129:823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis. 2015;30:1515–1529. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissier J, Chlichlia K, Digon Y, Ruppel A, Mone H. Preliminary study on sex-related inflammatory reactions in mice infected with Schistosoma mansoni. Parasitol Res. 2003;91:144–150. doi: 10.1007/s00436-003-0943-1. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161:5987–5996. [PubMed] [Google Scholar]
- Burrows G, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark A. Design, engineering, and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- Canazza A, Minati L, Boffano C, Parati E, Binks S. Experimental models of brain ischemia: a review of techniques, magnetic resonance imaging, and investigational cell-based therapies. Front Neurol. 2014;5:19. doi: 10.3389/fneur.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals JB, Pieri NC, Feitosa ML, Ercolin AC, Roballo KC, Barreto RS, Bressan FF, Martins DS, Miglino MA, Ambrosio CE. The use of animal models for stroke research: a review. Comp Med. 2011;61:305–313. [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hurn PD. Sex shapes experimental ischemic brain injury. Steroids. 2010;75:754–759. doi: 10.1016/j.steroids.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:1–15. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- Chu M, Hu X, Lu S, Gan Y, Li P, Guo Y, Zhang J, Chen J, Gao Y. Focal cerebral ischemia activates neurovascular restorative dynamics in mouse brain. Front Biosci. 2012;4:1926–1936. doi: 10.2741/513. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Conway SE, Roy-O’Reilly M, Friedler B, Staff I, Fortunato G, McCullough LD. Sex differences and the role of IL-10 in ischemic stroke recovery. Biol Sex Differ. 2015;6:17. doi: 10.1186/s13293-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70:1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- Davis CM, Fairbanks SL, Alkayed NJ. Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res. 2013;4:381–389. doi: 10.1007/s12975-012-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinal. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Casalena G, Jia J, Sultana R, Barone E, Cai J, Pierce WM, Cini C, Mancuso C, Perluigi M, Davis CM, Alkayed NJ, Butterfield DA. Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem. 2012;121:680–692. doi: 10.1111/j.1471-4159.2012.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Zhu W, Libal N, Alkayed NJ, Offner H. Different immunological mechanisms govern protection from experimental stroke in young and older mice with recombinant TCR ligand therapy. Front Cell Neurosci. 2014;8:284. doi: 10.3389/fncel.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Chen Y, Zhu W, Libal N, Alkayed NJ, Offner H. Partial MHC constructs treat thromboembolic ischemic stroke characterized by early immune expansion. Transl Stroke Res. 2016a;7:70–78. doi: 10.1007/s12975-015-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Chen Y, Manning D, Nguyen H, Saugstad JA, Offner H. Sex differences and the role of PPAR alpha in experimental stroke. Metab Brain Dis. 2016b;31:539–547. doi: 10.1007/s11011-015-9766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Liang J, Nguyen H, Manning D, Saugstad JA, Offner H. Loss of PPARalpha perpetuates sex differences in stroke reflected by peripheral immune mechanisms. Metab Brain Dis. 2016c;31:683–692. doi: 10.1007/s11011-016-9805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Dev Ther. 2015;9:3445–3454. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43:1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol. 2012;32:1936–1942. doi: 10.1161/ATVBAHA.112.251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova MA, Johnson MH, Ergul A, Hill WD, Hess DC, Sazonova IY. Sex-independent neuroprotection with minocycline after experimental thromboembolic stroke. Exp Transl Stroke Med. 2011;3:16. doi: 10.1186/2040-7378-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol. 2004;172:4556–4566. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalouli M, Carlsson L, Ameen C, Linden D, Ljungberg A, Michalik L, Eden S, Wahli W, Oscarsson J. Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology. 2003;144:101–109. doi: 10.1210/en.2002-220630. [DOI] [PubMed] [Google Scholar]
- Jones DC, Ding X, Daynes RA. Nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J Biol Chem. 2002;277:6838–6845. doi: 10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport. 1994;5:2481–2485. doi: 10.1097/00001756-199412000-00019. [DOI] [PubMed] [Google Scholar]
- Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Wiendl H. Con: regulatory T cells are protective in ischemic stroke. Stroke. 2013;44:e87–e88. doi: 10.1161/STROKEAHA.113.001268. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral micro-vasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Llovera G, Liesz A. The next step in translational research: lessons learned from the first preclinical randomized controlled trial. J Neurochem. 2016 doi: 10.1111/jnc.13516. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lofmark U, Hammarstrom A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Results from a community-based incidence study in northern Sweden. Neuroepidemiology. 2007;28:135–141. doi: 10.1159/000102141. [DOI] [PubMed] [Google Scholar]
- Losey P, Ladds E, Laprais M, Geuvel B, Burns L, Bordet R, Anthony DC. The role of PPAR activation during the systemic response to brain injury. J Neuroinflammation. 2015;12:99. doi: 10.1186/s12974-015-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusardi TA, Murphy SJ, Phillips JI, Chen Y, Davis CM, Young JM, Thompson SJ, Saugstad JA. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front Mol Neurosci. 2014;7:11. doi: 10.3389/fnmol.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health. 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42:595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Neyer J, Greenlund K, Denny C, Keenan N, Casper M, Labarthe D, Croft J. Prevalence of stroke—United States, 2005. JAMA. 2007;298:279–281. [Google Scholar]
- Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3+ Treg cells and gender bias in autoimmune diseases. Front Immunol. 2015;6:493. doi: 10.3389/fimmu.2015.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a phase I clinical study. J Neuroimmunol. 2011;231:7–14. doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro NI, Kane SV. Gender-related issues in the female inflammatory bowel disease patient. Expert Rev Gastroenterol Hepatol. 2009;3:145–154. doi: 10.1586/egh.09.1. [DOI] [PubMed] [Google Scholar]
- Onwuekwe I, Ezeala-Adikaibe B. Ischemic stroke and neuroprotection. Ann Med Health Sci Res. 2012;2:186–190. doi: 10.4103/2141-9248.105669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski RP, Schulte RW, Nie Y, Ling T, Lee T, Manaenko A, Gridley DS, Zhang JH. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res. 2012;3:473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk T, Potey C, Gautier S, Bastide M, Deplanque D, Staels B, Duriez P, Leys D, Bordet R. PPARs: a potential target for a disease-modifying strategy in stroke. Curr Drug Targets. 2013;14:752–767. doi: 10.2174/1389450111314070005. [DOI] [PubMed] [Google Scholar]
- Ouk T, Gautier S, Petrault M, Montaigne D, Marechal X, Masse I, Devedjian JC, Deplanque D, Bastide M, Neviere R, Duriez P, Staels B, Pasquier F, Leys D, Bordet R. Effects of the PPAR-alpha agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab. 2014;34:542–551. doi: 10.1038/jcbfm.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG American Heart Association Advocacy Coordinating Committee, Stroke Council. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- Pan J, Palmateer J, Schallert T, Hart M, Pandya A, Vandenbark AA, Offner H, Hurn PD. Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke. Transl Stroke Res. 2014;5:577–585. doi: 10.1007/s12975-014-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Offner H. The role of the spleen in ischemic stroke. J Cereb Blood Flow Metab. 2014;35:186–187. doi: 10.1038/jcbfm.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-de Puig I, Miro F, Salas-Perdomo A, Bonfill-Teixidor E, Ferrer-Ferrer M, Marquez-Kisinousky L, Planas AM. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J Cereb Blood Flow Metab. 2013;33:1955–1966. doi: 10.1038/jcbfm.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nat Med. 2009;15:138–139. doi: 10.1038/nm0209-138. [DOI] [PubMed] [Google Scholar]
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Sahota P, Vahidy F, Nguyen C, Bui TT, Yang B, Parsha K, Garrett J, Bambhroliya A, Barreto A, Grotta JC, Aronowski J, Rahbar MH, Savitz S. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke. 2013;8:60–67. doi: 10.1111/ijs.12022. [DOI] [PubMed] [Google Scholar]
- Schabitz WR. Regulatory T cells in ischemic stroke: helpful or hazardous? Stroke. 2013;44:e84. doi: 10.1161/STROKEAHA.113.002228. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012a;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012b;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: possible molecular mechanisms. J Neurosci Res. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: the heart and estrogen-progestin replacement study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33:37–47. doi: 10.1038/jcbfm.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, Huber JD. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sci. 2013;14:1890–1917. doi: 10.3390/ijms14011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Meza-Romero R, Benedek G, Andrew S, Huan J, Chou YK, Buenafe AC, Dahan R, Reiter Y, Mooney JL, Offner H, Burrows GG. A novel regulatory pathway for autoimmune disease: binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. J Autoimmun. 2013;40:96–110. doi: 10.1016/j.jaut.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacres MC, Longmate J, Auge C, Diamond DJ. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol. 2004;65:476–485. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Alexander SI. CD8 regulatory T cells: what’s old is now new. Immunol Cell Biol. 2009;87:192–193. doi: 10.1038/icb.2009.8. [DOI] [PubMed] [Google Scholar]
- Weinstein Y, Ran S, Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol. 1984;132:656–661. [PubMed] [Google Scholar]
- Xia HJ, Zhang GH, Wang RR, Zheng YT. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol Immunol. 2009;6:433–440. doi: 10.1038/cmi.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026–2032. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG. IL-4 is required for sex differences in vulnerability to focal ischemia in mice. Stroke. 2015;46:2271–2276. doi: 10.1161/STROKEAHA.115.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. ScientificWorldJournal. 2013;2013:174373. doi: 10.1155/2013/174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Davis CM, Edin ML, Lee CR, Zeldin DC, Alkayed NJ. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS One. 2013;8:e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyab AH, Raza A, Karmaus W, Tongue N, Zhang H, Matthews S, Arshad SH, Roberts G. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy. 2010;40:1776–1784. doi: 10.1111/j.1365-2222.2010.03633.x. [DOI] [PubMed] [Google Scholar]