Abstract
CD98hc (SLC3A2) is the heavy chain component of the dimeric transmembrane glycoprotein CD98, which comprises the large neutral amino acid transporter LAT1 (SLC7A5) in cells. Overexpression of CD98hc occurs widely in cancer cells, and is associated with poor prognosis clinically, but its exact contributions to tumorigenesis are uncertain. In this study, we showed that that genetic deficiency of CD98hc protects against Ras-driven skin carcinogenesis. Deleting CD98hc after tumor induction was also sufficient to cause regression of existing tumors. Investigations into the basis for these effects defined two new functions of CD98hc that contribute to epithelial cancer beyond an intrinsic effect on CD98hc on tumor cell proliferation. First, CD98hc increased the stiffness of the tumor microenvironment. Second, CD98hc amplified the capacity of cells to respond to matrix rigidity, an essential factor in tumor development. Mechanistically, CD98hc mediated this stiffness-sensing by increasing Rho kinase (ROCK) activity, resulting in increased transcription mediated by YAP/TAZ, a nuclear relay for mechanical signals. Our results suggest that CD98hc contributes to carcinogenesis by amplifying a positive feedback loop which increases both extracellular matrix stiffness and resulting cellular responses. This work supports a rationale to explore the use of CD98hc inhibitors as cancer therapeutics,
Keywords: CD98hc/SLC3A2, Ras-driven cancer, gain-amplifier, stiffness, YAP/TAZ
Introduction
Skin squamous cell carcinoma (SCC) is the second most common skin cancer (1). Expression of the type II transmembrane glycoprotein CD98 heavy chain (4F2, SLC3A2) is highly increased in most carcinomas, including SCC, as well as transformed cell lines (2), while in skin, CD98hc is required for proper homeostasis and efficient epidermal wound closure. Via its extracellular domain (C109), CD98hc covalently binds one of several catalytic subunits (the SLC7A5–11 family) to form the functional Heteromeric Amino acid Transporters (HAT) at the cell surface (3). Besides its role as amino acid transport mediator, CD98hc, by binding to intracellular domains to the adhesion receptor β1 integrins, enhances integrin outside-in signaling (4), thus modulating integrin-dependent cell functions.
CD98hc over expressing NIH-3T3 fibroblasts developed malignant tumors in athymic mice (5,6). We previously showed that CD98hc deletion in mouse embryonic stem (ES) cells delays teratocarcinoma formation in mice (4). CD98hc is an integrin binding protein that enhances signals downstream of β1 integrin therefore regulating integrin-dependent cell behavior, including matrix remodeling in vitro (4,7,8). Tumorigenicity of CD98hc deficient ES cells can be reconstituted by expressing a chimeric form of CD98hc which is able to interact with β1 integrin (4). This CD98hc/β1 interaction contributes to cell transformation in vitro (9).
Here we examine the role of CD98hc in a well-established (10) two step model of squamous cell carcinoma (SCC). In this Ras-driven cancer model, tumorigenesis begins with the initiation of a single epidermal cell, which occurs following a single subcarcinogenic dose of 7,12-dimethylbenz[a]-anthracene (DMBA). Following the initiation stage, the population of mutated cells is promoted to clonally expand during the second stage, referred to as “promotion”. Tumor promotion is elicited by the repeated topical application of chemical agents, such as the phorbol ester, phorbol 12-myristate 13-acetate (TPA) that leads to sustained epidermal hyperplasia as evidenced by an increase in the number of nucleated cell layers and an overall increase in thickness of the epidermis. Tumorigenesis proceeds through the promotion of benign tumors (papilloma) growth and finally the progression of some benign tumors into malignant and potentially metastatic lesions (SCC). This two-stage skin carcinogenesis model enables direct visualization of tumor development and permits evaluation of TPA-induced inflammation response (10).
Here, we combined this model of epidermal tumor formation with conditional deletion of CD98hc in basal keratinocytes to reveal a major contribution of CD98hc in controlling the mechanical properties of the tumor microenvironment, independently of skin TPA-induced inflammation. Specifically, CD98hc deficient skin was protected against tumor formation, and that CD98hc deletion led to regression of pre-existing tumors. We demonstrate that, beyond CD98hc intrinsic proliferation effect within tumor cells, CD98hc-expressing environment is cancer-prone due to modulation of its mechanical properties. This model is known to be sensitive to integrin signaling and rigidity sensing. We now show that CD98hc acts as a gain amplifier for stiffness sensing via integrins. CD98hc is thus a gain amplifier of a positive feedback loop that increases ROCK signaling, matrix stiffness and YAP/TAZ-driven gene expression.
Material and Methods
Mice
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nice-Sophia Antipolis, Nice, FR (CIEPAL-AZUR Agreement NCE/2012-66). K14-CreERT2, CD98hcfl/fl have been described previously (11). The experiments have been performed on pure FVB/n background mice.
Chemical Carcinogenesis
Chemical carcinogenesis experiments were performed on 10-week-old mice (20 animals/group), essentially as previously described (10). Mice received one topical application of 200 nmol of 7,12-dimethylbenz(a)anthracene (DMBA; Sigma-Aldrich) in 200 µL of acetone followed a week later by biweekly applications of 6.8 nmol of phorbol 12-myristate-13-acetate (TPA; Sigma-Aldrich) in 200 µl acetone. Papillomas and SCC numbers were scored once a week for up to 32 weeks after the start of promotion. Topical 4-OHT treatment was topically applied when indicated (6 treatments of 1.5 mg 4OHT in ethanol/back). No difference in benign tumor acquisition was observed in 4-OHT-treated wt mice ruling out any possible effects of 4-OHT treatment (data not shown).
TPA-induced skin response
Ears (both sides) of mice with WT- or CD98hc-deficient skin were topically treated with 20µl (per side) of either TPA (0.5µg per side) or vehicle (acetone). Ear thickness was measured with digital calipers, and ear swelling was calculated as (thickness at each time point)-(thickness at 0h). Paraffin sections were stained with H&E and granulocytes were quantified using ImageJ software.
Histology and Immunohistochemistry
Samples were collected as described by Montanez et al. (12). Histology and immunochemistry were performed as described previously (11). Paraffin-embedded sections were used for all stainings except CD98hc immunostaining, which was performed on frozen sections. Primary antibodies were: rabbit anti human P-cofilin (Cell Signalling), rabbit anti human P-MYPT1 (Millipore), rabbit anti human MYPT1 (Millipore), rat anti mouse CD98 (Clone RL388, e bioscience). Secondary antibodies were used according to manufacturer’s instructions, for immunofluorescence Alexa Fluor 594 anti Rat # A- 21209 anti rabbit #A-21207 (Life technologies); and for immunohistochemistry Vectastain ABC-Kit (Vector #4001) with DAB reagent (Vector #SK4100).
Elastic Modulus Measurements
To carry out topography imaging and simultaneous elastic modulus maps, a Bioscope Catalyst operating in Peak Force Quantitative Nanomechanical Mapping mode (Bruker Nano, Inc.), coupled with an inverted optical microscope (DMI 6000B, Leica), was used. The experiments were performed using V-shaped silicon SNL-D probes (nominal spring constant k= 0.06 N/m, side angle 23 °, Bruker Nano, Inc.). For each sample (10µm unfixed frozen sagittal section (13), a peak force of 5 nN and a tip velocity of 4 micron/s were used (detailed procedure in Supp. Mat. & Methods). For collagen deposition analysis, detailed procedure is in Supp. Mat. & Methods.
Cells
Mouse embryonic fibroblasts (MEFs) were derived from CD98hc-conditional knockout homozygote embryos as described previously (7). Similarly, murine keratinocytes were generated and cultured from backskin of adult mice as described in (11) (culture conditions, see Supp. Data). Both cell types were identified by PCR on genomic DNA (as for mice (7), CD98hc expressing or deficient lines), which was performed regularly during their use. MEFs were maintained in DMEM-H (Invitrogen) culture medium containing 10% (v/v) FBS (HyClone), 20 mM HEPES, pH 7.3 (Invitrogen), 0.1 mM nonessential amino acid (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), and 2 mM L-Glutamine (Invitrogen) at 37°C and 5% CO2. The CD98hc-null MEFs were generated by infecting CD98hc floxed MEFs with adeno-CRE encoding CRE recombinase.
Cell Spreading Assay
MEFs were replated on the fibronectin-coated gels (PDMS, Supp. Mat. & Methods, or for Supp. Fig. S3, Polyacrylamide, Matrigen, CA, USA) with indicated stiffness and incubated in DMEM plus 1% (v/v) BSA for 45 min, or grown on gels for 24 hours prior to treatment with 1µg/ml of either Rho inhibitor I or Rho activator II (Cytoskeleton, CO, USA) in serum-free medium for 4 hours. Alternatively, CD98hc-null MEFs infected with retroviruses encoding wild type CD98hc, wild type CD69 or chimeras (C69T98E98, C98T69E98, or C98T98E69) were replated on 30 kPa of PDMS gels for 45 min in the absence of serum. Cells were fixed with 3.7% formaldehyde in PBS, permeabilized with 0.5% Triton X-100 in PBS at room temperature (RT) for 10 min, and washed three times with PBS. The cells were then incubated with phalloidin-conjugated rhodamine (Molecular Probes) for 1 hr at RT. The cells on coverslips were washed three times with PBS, mounted with a mounting solution (ProLong® Gold antifade reagent; Invitrogen), and visualized by fluorescent microscopy (Eclipse TE2000-U, Nikon). Cell spreading was quantified by cell area by using customized software written in MATLAB (MathWorks).
Western Blotting
MEFs were replated on PDMS gels as described above. When mentioned, CD98hc-null MEFs were transiently transfected with wild type CD98hc, extracellular domain-deleted CD98hc or CD69 for 36hr then replated on 30 kPa PDMS substrates and incubated as above. Whole cell lysates were prepared using modified radioimmune precipitation assay buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 50 mM NaF, 1 mM sodium pyrophosphate, 0.1% sodium deoxycholate, 1% Nonidet P-40, protease inhibitors cocktail and 1% CHAPS). Protein lysates were quantified using the bicinchoninic acid (BCA) method (Thermo Scientific Pierce), loaded on SDS-PAGE, and analyzed by Western blotting. The primary antibodies for western blotting were anti-phospho-Y576FAK (Invitrogen), anti-FAK (Santa Cruz Biotechnology), anti-phospho-S476Akt (Cell Signaling Technology), anti-Akt (Cell Signaling Technology) and anti-α-tubulin (Sigma Aldrich).
qPCR
RNAs were extracted from back skin samples (bearing or not tumors) using Trizol reagent (GIBCO), or from MEFs plated on gels using RNeasy plus kit (Qiagen),. Reverse transcription was performed on total RNA using Superscript II reverse transcriptase (Invitrogen) according to manufacturer’s instructions. Sets of specific primers (see supplemental Table 1) were used for amplification using 7900HT Real Time PCR System (AppliedBiosystems, Foster USA). Samples were normalized to GAPDH (tumors) or rplp0 (MEFs) using the ΔCt method. Statistical significance was determined with Student’s t-test.
Results
CD98hc deficiency inhibits Ras-induced tumorigenesis
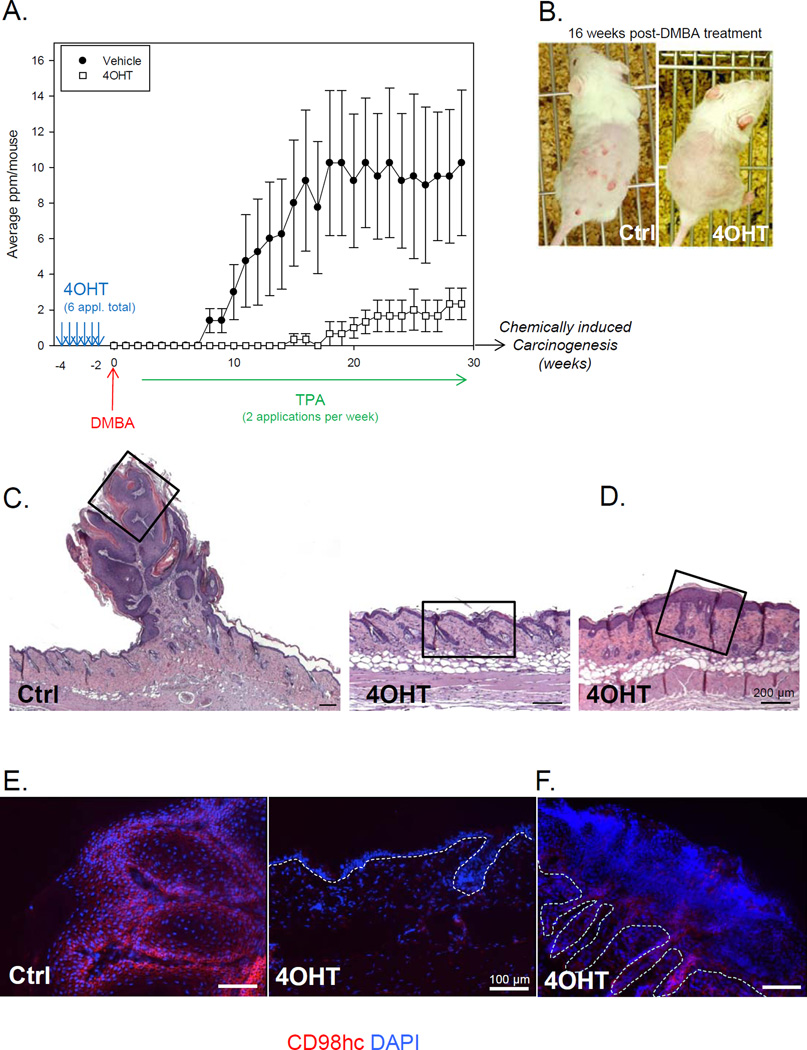
CD98hc expression is linked to malignancy (14,15,2) and CD98hc can regulate tumor-associated processes such as anchorage independence (8,4), nutrient transport (3,16), and mTOR (17). To assess CD98hc function in skin tumorigenesis, we generated mice with conditional deletion of CD98hc in the basal layer of the epidermis (K14CreERT2;CD98fl/fl) (11) and applied a two-stage chemical skin carcinogenesis protocol. Topical treatment with the carcinogen DMBA induced oncogenic Ras mutations, and subsequent repeated treatments with the phorbol ester TPA promoted outgrowth of initiated cells resulting in benign papillomas in control mice about 8 weeks after initiation (average of 3 papillomas/mouse at 10 weeks and 10 papillomas/mouse by 16 weeks) (Fig. 1B and 1C). Strikingly, papilloma formation was delayed by 8 weeks in CD98hc-deficient mice. Even after 25 weeks of TPA treatment, only 2 papillomas/mouse, on average, were detected on CD98hc deficient skin, whereas 10/mouse were observed in control mice (Fig. 1A and 1B). Tumor multiplicity was strongly reduced (Fig. 1A), suggesting that CD98hc promotes Ras-induced tumor formation and growth. Typical papillomas in vehicle-treated mice showed exophytic growth of squamous cells. In contrast, 4OHT-treated skin only occasionally manifested hyperplasia of the epidermis (Fig. 1D). CD98hc deletion was induced by 2 week-pretreatment with 4-hydroxy-tamoxifen (4OHT) compared to vehicle-treated skin (Fig. 1A and 1E). The rare papillomas or hyperplastic area, observed on skin treated with 4OHT, expressed CD98hc, (Fig. 1F), suggesting that they were formed by keratinocytes that had escaped Cre-mediated recombination. These data strongly suggest a tumor-promoting function of CD98hc in Ras-induced papillomas.
Figure 1. CD98hc deficient epidermis is protected against tumor formation in vivo.
A. Number of papillomas per mouse is shown over the course of the experiment. Mice (8 weeks old) were treated 6 times with 4OHT before the start of the chemical carcinogenesis (DMBA/TPA) protocol. Data are mean (±SEM) of 2 independent experiments. (n=10 per group). B. Representative pictures at 16 weeks post initiation of mice from vehicle- (Ctrl) and 4OHT-treated group. C. Back skin sections, stained with H&E, isolated from vehicle- (Ctrl) or 4OHT-treated mice (as indicated) after 30 weeks of promotion, bearing or not tumors. Inserts are shown in D and E. Scale bar 200µm. D, E. Immunofluorescence analysis of skin samples from vehicle- (Ctrl) or 4OHT-treated mice (as indicated) with antibody against CD98hc. Dotted line indicates the dermis-epidermis junction. Scale bar 100µm.
TPA-associated inflammatory response in the skin is sustained in the absence of CD98hc
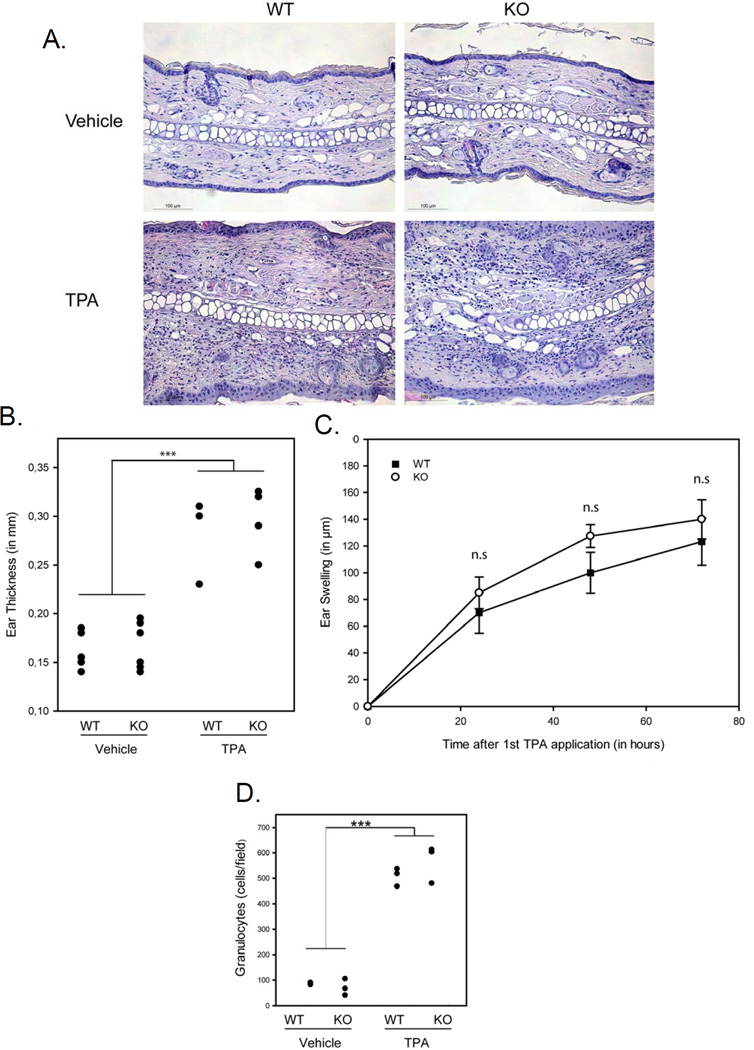
Inflammation facilitates cancer development and growth. Because intestinal epithelia CD98hc is required for inflammation-induced tumorigenesis in the gut, promoting colitis-associated cancer in mice (15), we analyzed the effect of epidermal CD98hc loss in the inflammatory responses stimulated by topical TPA application, the known inflammatory inducer in skin model. First, we quantified the extent of skin edema by treating the ears with TPA and measuring their thickness. A strong reaction was observed following TPA application (vs. vehicle treated skin)(Fig. 2A and 2B), histologic analysis of the ears, prepared 6h after last TPA application, revealed no gross difference in ear thickening and swelling of WT versus CD98hc KO mice (Fig. 2A, 2B, and 2C). Besides, marked spongiosis and extensive infiltration of leukocytes were observed in the edematous dermis independent of CD98hc expression (Fig. 2A). Similar TPA-induced infiltration of inflammatory leukocytes was detected between CD98hc expressing and deficient mice (Fig. 2D). Thus, TPA-induced inflammatory response, ultimately leading to skin tumorigenesis, is CD98hc-independent. Altogether, these data strongly suggest that the dramatic effect of CD98hc loss on skin tumorigenesis relates to a cell autonomous effect.
Figure 2. TPA-induced inflammatory response is preserved in absence of CD98hc.
A. Measurement of TPA-induced ear swelling. Ears of CD98hc- expressing or deficient mice were treated with TPA or vehicle alone. Seventy two hours after the first treatment, ears were collected. H&E stainings of representative ear cross sections are shown. Bar 100µm. B. Ear thickness of each mouse is shown (black dot represents value for each mouse). *** p<0.001 between TPA vs. vehicle. C. Time course of TPA-induced ear swelling. Extend of ear swelling was calculated as indicated in Mat. & Meth (n=3 per group). No significant difference was observed in the presence or absence of epidermal CD98hc. D. Quantification of the densities of infiltrated granulocytes of the ear of each mouse 72 hours after the first TPA treatment (each cross sections, as represented in A, were quantified), black dot represents value for each mouse). *** p<0.001 between TPA vs. vehicle.
Loss of CD98hc causes Ras mutation-bearing tumor regression
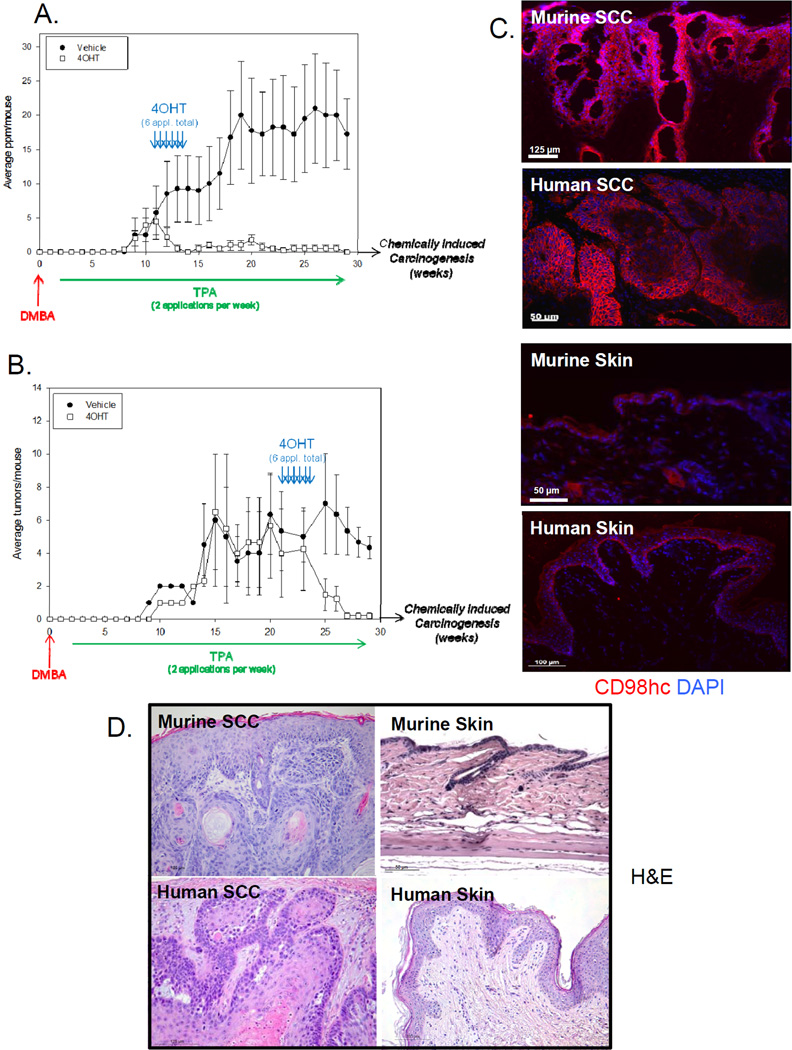
Because CD98hc has been shown to act intrinsically as cell proliferation enhancer both in tumoral and non-tumoral cells, Ras-induced tumorigenesis inhibition in CD98hc-deficient skin was expected. Thus, we analyzed the effect of CD98hc deficiency once tumors were already formed. Following either 11 or 23 weeks of DMBA/TPA (inducing respectively, early or large papillomas defined as a size of over 6mm (before SCC conversion), we treated mice with topical 4OHT or vehicle, and monitored succeeding tumor progression (Fig. 3A and 3B). In both cases and within 2 weeks (4OHT treatment period), not only further papilloma growth or conversion to SCC were totally abrogated, in spite of continued TPA application, but papillomas fully regressed, irrespective of papilloma initial size (Fig. 3A and 3B, regression within 5–7 weeks). In sharp contrast, in the first set-up, vehicle-treated mice exhibited progressive papilloma formation such that, at 19 weeks, they manifested an average of 18/mouse (Fig. 3A). In the second set-up, by 27 weeks post DMBA, SCC conversion occurred only in CD98hc expressing mice, with an average of one SCC per mouse, as judged by their flattened morphology and downward-appearing growth (Fig. 3B). Besides, these SCCs exhibited markedly increased CD98hc expression as compared to normal skin and a similar increased expression was observed in human SCC (Fig. 3C). The development of SCC in control mice was confirmed by histological analysis (Fig. 3D). Furthermore, increased CD98hc expression was a general feature of human SCC, being present in all 12 human SCC studies (GEO Profiles for SLC3A2), compiled over 310 samples. Thus, while CD98hc ablation in skin does not modulate apoptosis (11), it induces a drastic regression of Ras mutation-bearing tumors, preventing progression to SCC.
Figure 3. CD98hc loss induces tumor regression.
A. Number of papillomas per mouse is shown over the course of the experiment. Mice (8 weeks old) were subjected to the chemical carcinogenesis protocol DMBA/TPA. Six 4OHT-(or vehicle-) treatments were applied on the back skin of treated group 12 weeks after the DMBA application, corresponding to the first papillomas apparition. Data are mean (±SEM) of two independent experiments. n=6 per group. B. Number of tumors per mouse is shown over the course of the experiment. Mice (8 weeks old) were subjected to the chemical carcinogenesis protocol DMBA/TPA. Six 4OHT- (or vehicle-) treatments were applied on the back skin of treated group 22 weeks after the DMBA application, corresponding to papilloma conversion to malignant SCC. Only large papillomas, defined as a size of over 6mm, are shown. Data are mean ±SEM representative of n=10 for vehicle-treated (Ctrl) group and n=14 for 4OHT-treated group. C. Immunofluorescence analysis of SCC (upper panels) and normal skin (lower panels) samples from either murine or human origin with antibody against CD98hc, showing strong overexpression of CD98hc in SCC. Scale bars as indicated. D. Morphology of SCC tumor (left panel) and normal skin (right panel) sections shown by H&E staining. Scale bars as indicated.
CD98hc regulates the elasticity of the tumor microenvironment
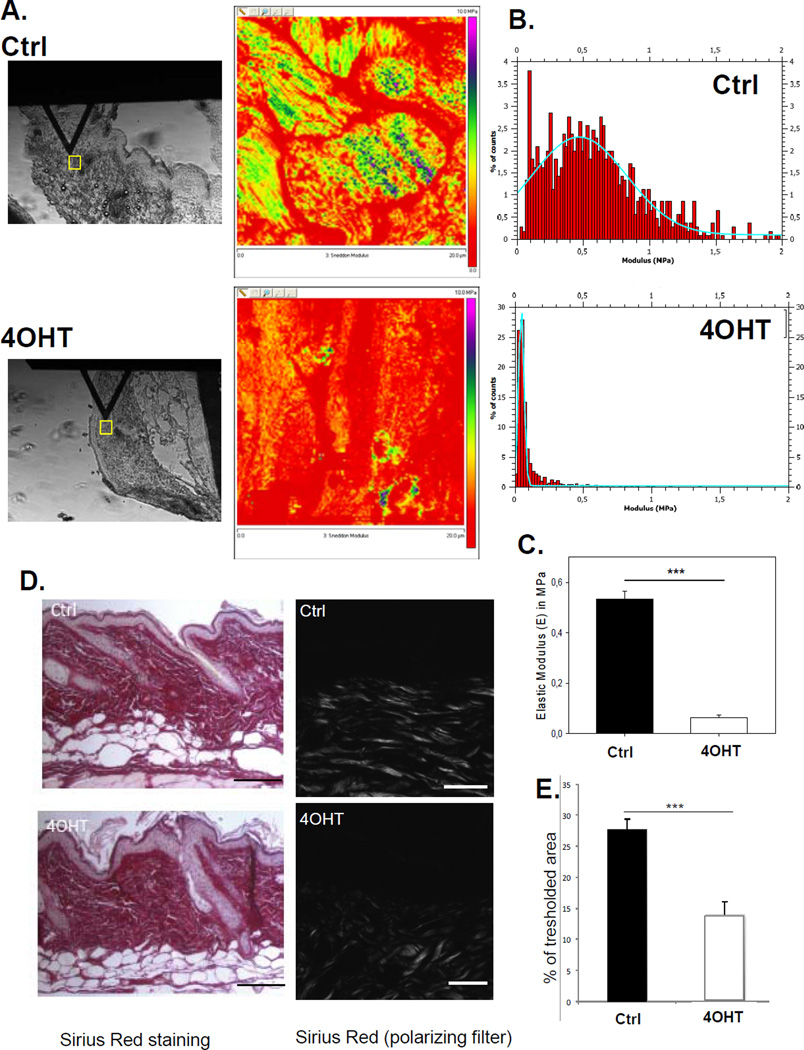
To assess how CD98hc deficiency impacts tumorigenesis, beyond its cell autonomous effect on proliferation and absence of extrinsic effect on TPA-induced inflammation, we analyzed mechanical features of the tumor microenvironment. A stiff and organized 3D microenvironment is important in tumor formation and tumor cell invasiveness (18) and we previously showed that, in fibroblasts, CD98hc can regulate RhoA (7), an important regulator of skin stiffness (13). Interestingly, CD98hc-deleted skin exhibited an atomic force microscopy (AFM) force map skewed toward low MPa values indicating more compliant tissue (Fig. 4A, 4B and Supp. Fig.S1A). The AFM nanoindentation of the epidermal CD98hc deficient skin revealed an elastic modulus of 0.063±0.01 MPa (n=4), while wt skin, elastic modulus in the papillary (upper) dermis reached 0.53±0.03 MPa (mean, n=3) (Fig. 4C). Although other methods have reported varying E values (19), (20), ’(21), our data are in good agreement with previous AFM studies (22) performed on skin. Thus, CD98hc deficiency decreases epidermal tissue stiffness. Highly aligned ECM molecules, in particular collagen fibers, increase matrix strength and stiffness in vivo. Skin sections of control and 4OHT treated mice stained with Masson’s trichrome (Supp. Fig.S1B) and sirius red (Fig. 4 D, 4E) highlight a major defect in fibrillar collagen, as seen using polarized filter, with a less organized collagen-rich dermis in CD98hc deficient skin (Supp. Fig.S1C–D). Similarly, abnormal fibronectin deposition was observed in KO skin. Therefore, we conclude that the loss of epidermal CD98hc results in more compliant skin due to drastic in vivo changes on collagen and fibronectin organization.
Figure 4. Loss of CD98hc increases tissue elasticity by modulating ECM organization.
A. AFM maps of the elastic moduli of vehicle- (Ctrl) and 4OHT-treated skin corresponding to the areas enclosed by yellow squares (left panels). Right panels show heat maps of the same 20 × 20 µm scanned areas. B. Representative force-separation curves of vehicle (Ctrl) and 4OHT -treated samples. C. Quantification of Young’s elastic Modulus in vehicle (n=3) and 4OHT (n=4) treated skin, expressed as mean ± SEM, ***p value<0.001). D. Defect in fibrillar collagen in 4OHT-treated skin shown by sirius red staining (right panel corresponds to polarized filter which highlights fibrillar collagen). Scale bars 100µm and 50 µm, respectively. E. Quantification of fibrillar collagen in 4OHT- vs. vehicle- (Ctrl) treated skin confirming a less organized collagen-rich dermis in CD98hc deficient skin.
CD98hc-mediates increased ROCK and YAP/TAZ signaling during tumorigenesis
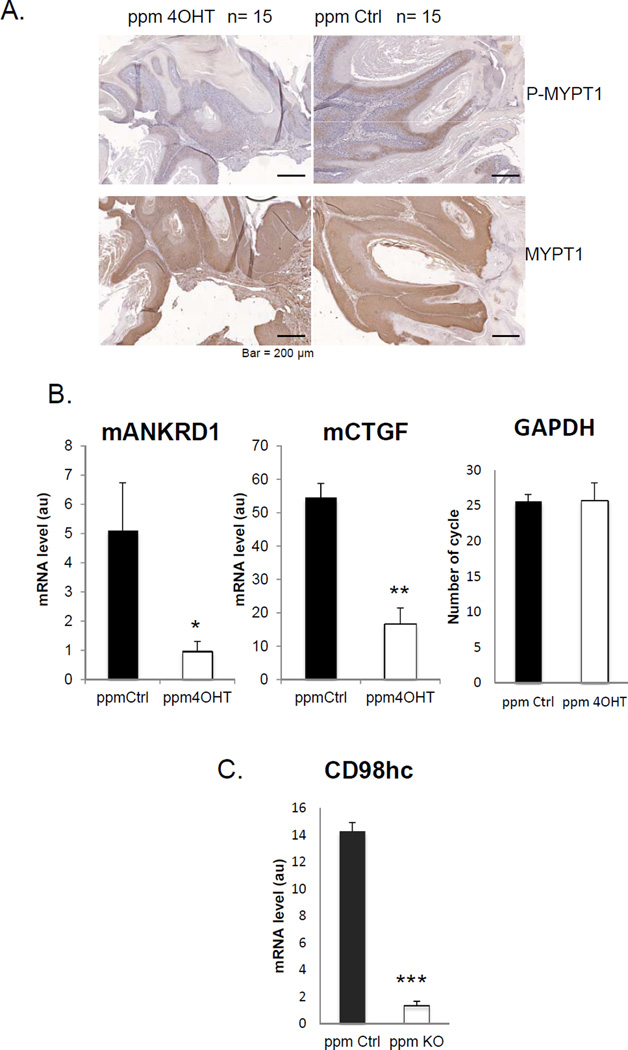
There is an intimate and bidirectional relationship between matrix organization/stiffness and RhoA-driven cellular contractility mediated by its effector Rho Kinase (ROCK) (13,19) : cellular contractility stimulates matrix assembly (23) and a stiffer matrix stimulates RhoA activity (24). We previously showed that, loss of CD98hc induces a reduction of RhoA activation in unchallenged skin (11). We therefore assessed the effect of CD98hc deletion in keratinocytes on RhoA signaling in papillomas as judged by the ROCK-mediated phosphorylation of the regulatory subunit of myosin phosphatase (Mypt) (Fig. 5A). Papillomas exhibited extensive phospho-Mypt in the epithelium, particularly in suprabasal layers. In contrast phospho-Mypt was nearly absent in the few papillomas derived from 4OHT-treated Slc3a2fl/fl-K14CreERT2 mice (Fig. 5A). Similarly, phosphorylation of cofilin, an indirect downstream target of ROCK, was lost in 4OHT-treated- compared to vehicle treated-skins (phospho-cofilin staining, Supp. Fig. S2). Thus, in contrast to wound healing with a soft provisional matrix (11), CD98hc deletion markedly reduced ROCK activity in papillomas associated with a reduction in matrix stiffness.
Figure 5. CD98hc loss reduces Rho Kinase (ROCK) activity in papillomas resulting in decreased YAP/TAZ Signaling.
A. Immunohistochemical analysis of murine papilloma sections from vehicle- (Ctrl) and 4OHT-treated mice with antibodies against MYPT1 and P-MYPT1, displaying strong expression across the epidermis, but decreased phosphorylation status in CD98hc deficient papillomas. Scale bar 200µm. B. Quantitative RT-PCR of YAP/TAZ signaling markers (mANKRD1 and mCTGF) from papilloma isolated from vehicle- (Ctrl) and 4OHT-treated mice. GAPDH is shown as control. Data shown are mean ±SEM (n=15 per group), *p< 0.01, **p≤0.001. C. Quantitative RT-PCR of CD98hc in vehicle- (Ctrl) vs. 4OHT-treated papillomas. (n=5 per group) Data are shown as mean ± SEM, ***p< 0.001.
ROCK regulates YAP/TAZ (Yes-associated protein/Transcriptional co-activator with PDZ-binding motif), which is an important transcriptional mechanism that controls production of fibrogenic factors, such as CTGF (connective tissue growth factor), in addition to controlling cell size, proliferation, and malignant transformation (25). Moreover, recent work shows that cells can “read” ECM cues as a function of the activity of YAP/TAZ through RhoA/ROCK signaling (25). We, therefore, analyzed the effect of CD98hc depletion on YAP/TAZ transcriptional activity by assaying expression of endogenous target genes. Real-time PCR on papillomas revealed that there was a major reduction in transcripts of both mANKRD1 and mCTGF (~5 and ~3 fold reduction respectively), two well characterized YAP/TAZ targets (25) in 4OHT-treated compared to vehicle treated mice (Fig. 5B). As expected, mANKRD1 and mCTGF were even more highly expressed in SCC reaching levels about 5 fold greater than in papillomas (Supp. Fig. S3A). Thus, loss of basal keratinocyte CD98hc markedly suppresses both RhoA/ROCK signaling and expression of YAP/TAZ regulated genes.
CD98hc is a gain amplifier of stiffness sensing that enables a RhoA-driven self-reinforcing feedback loop that increases matrix stiffness
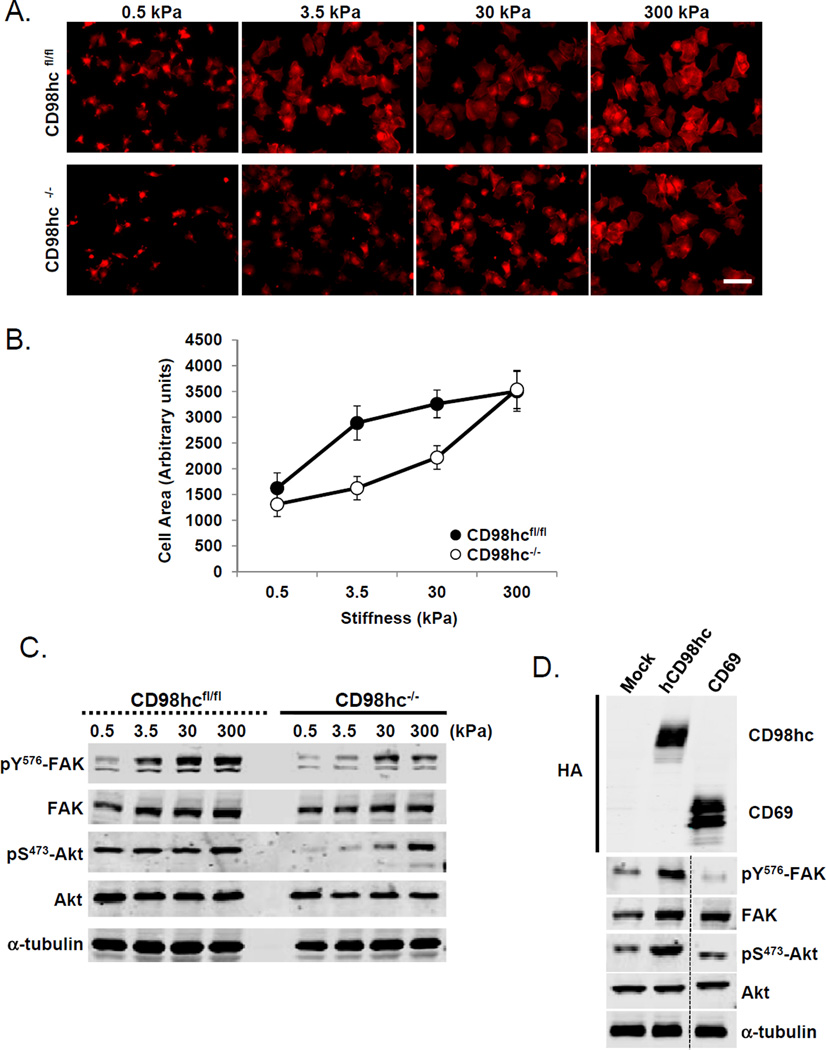
Integrins play a central role in stiffness sensing (26),,(27). We previously reported that CD98hc expression modulates integrin signaling which can influence the growth of teratocarcinomas (4). This suggests that CD98hc might alter the cellular responses to the tumor microenvironment. Cell spreading is controlled by extracellular matrix stiffness (28). CD98hc null cells exhibited a striking defect in stiffness sensing. Specifically, when adhering to fibronectin covalently bound to silicone gels of varying compliance, CD98hc null fibroblasts failed to spread on 3.5 kPa substrates, an elasticity in the range of those found in skin (19) (Fig. 6A and 6B). Similarly, activation of two integrin-regulated promoters of cell survival, proliferation, and tumor cell invasion, pp125FAK and AKT, was reduced (Fig. 6C). These defects were specifically rescued in cells expressing full length human CD98hc (Fig. 6D). Remarkably, adhesion to stiffer substrates (30 and 300 kPa), resulted in partial restoration of both cell spreading and biochemical signaling in the CD98hc null fibroblasts. Loss of CD98hc in vivo markedly suppresses RhoA/ROCK signaling resulting in decreased transcription of YAP/TAZ regulated genes (Fig. 5 and Supp. S3A). Direct activation of Rho with cytotoxic necrotizing factor-1e (CNF-1) in CD98hc-deficient cells rescued YAP/TAZ activation (Supp. Fig. S3B); conversely, its inhibition by exoenzyme C3 in WT cells blocked YAP/TAZ driven gene expression (Supp. Fig. S3C), thus we propose Actin-Rho/ROCK-matrix stiffness-YAP/TAZ cascade is critical downstream of CD98hc.
Figure 6. CD98hc is a central gain amplifier of stiffness sensing.
A. Actin staining of WT (CD98hcfl/fl) or CD98hc-deficient (CD98hc−/−) cells seeded on silicone gels displaying variable stiffness. Scale bar 20 µm. B. Quantification of cell area according to matrix stiffness. Data represented are mean of 3 independent experiments. Error Bars are SEM. C. Western blot analysis of FAK and Akt level of phosphorylation in WT or CD98hc-deficient cells according to matrix stiffness. Total FAK, Akt, and α -tubulin are shown as controls. D. Western blot analysis of FAK and Akt level of phosphorylation in CD98hc-deficient cells reconstituted with full length human CD98hc (hCD98hc) or CD69 (control). Total FAK, Akt, and α-tubulin are shown as controls.
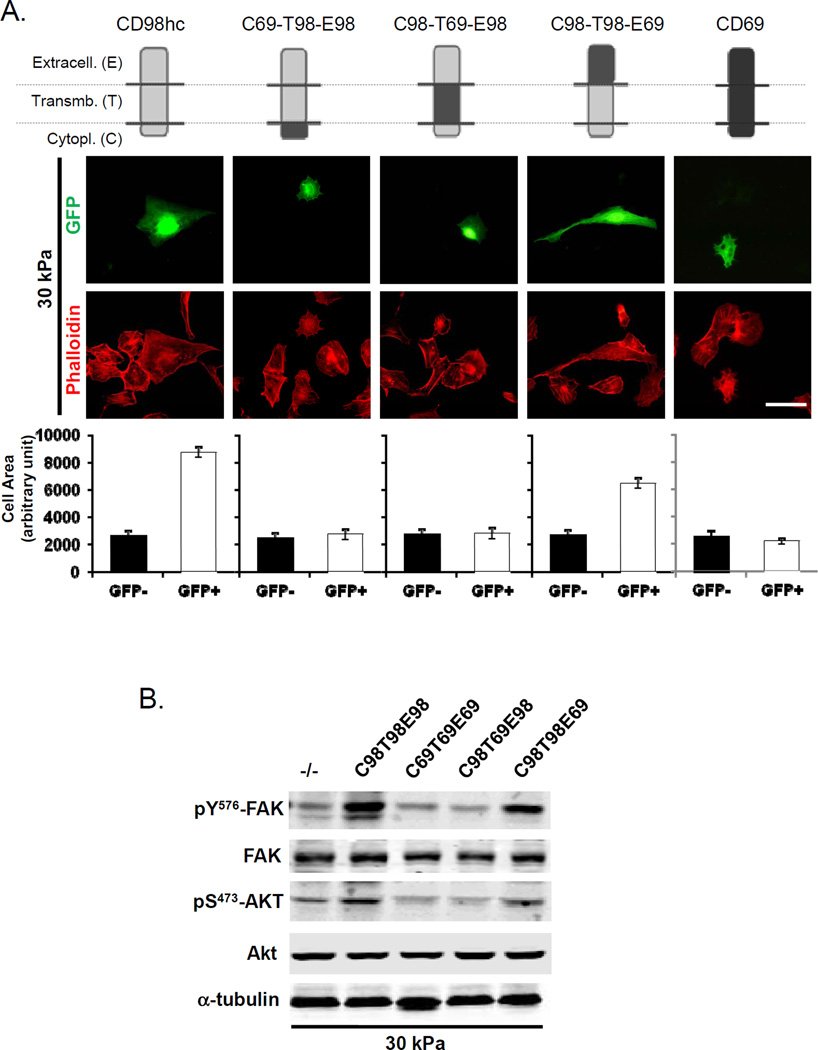
To assess whether effects on stiffness sensing were mediated through integrin interactions, we tested the capacity of reconstitution of the null cells with wild type CD98hc or with mutants (Fig. 7A and 7B). Both wild type and a mutant (C98T98E69) that couples only to integrins rescued the defect in stiffness sensing in the CD98hc fibroblasts. In contrast mutants that support amino acid transport but do not associate with integrins (C69T98E98 and C98T69E98) failed to rescue (Fig. 7A and 7B). Skin tumors arise from the underlying basal-layer cells, thus we tested the reconstitution of CD98hc deficient keratinocytes (Supp. Fig. S4) and found, similarly that, only mutant coupling with integrins (C98T98E69) rescued the defect in stiffness sensing. These data show that CD98hc, via its interaction with integrins, acts as a gain amplifier of stiffness sensing.
Figure 7. CD98hc capacity to stiffness sensing is mediated through integrin signaling.
A. CD98hc-deficient cells reconstituted with different chimeras (GFP positive) were seeded on 30 kPa stiffness silicone gels. Model of chimeras of CD98hc and another type II transmembrane protein (CD69), is shown. CD98hc protein is depicted in gray, and CD69 is depicted in black. Each chimera is defined by its cytoplasmic (C), transmembrane (T), and extracellular (E) domain derived from either CD98hc (98) or CD69 (69). CD98hc extracellular domain is necessary and sufficient for amino acid transport, whereas the intracellular and transmembrane domains are required for interactions with integrins. Actin cytoskeleton was observed using phalloidin staining. Quantification of cell surface area of transfected cells (GFP+) vs. Mock (GFP-), according to the matrix stiffness, is shown. Data represented are mean of 3 independent experiments. Error Bars are SEM. Scale bar 10 µm. B. Western blot analysis of FAK and Akt level of phosphorylation in CD98hc-deficient cells (CD98hc−/−) reconstituted with chimeras and seeded on 30 kPa silicone gels.
Discussion
In this study, we examined the sensitivity of the epidermal CD98hc-deficient mouse to Ras-driven skin carcinogenesis. Mice lacking epidermal CD98hc were protected against chemically-induced papilloma formation, even though TPA-associated inflammation response was preserved. Beyond this protection linked to CD98hc cell autonomous effect on cell proliferation, we demonstrate, for the first time, that when CD98hc deletion is induced after papillomas are formed, tumor regression is observed. Stiffness of the tumor microenvironment is crucial during cancer growth and integrins are involved in stiffness sensing. We reveal a new role for CD98hc as an amplifier of integrin-mediated cellular stiffness sensing leading to both ROCK-mediated increased matrix stiffness and nuclear relay of mechanical signals via YAP/TAZ signaling. Thus, CD98hc is a central gain amplifier in a self-reinforcing feedback loop that regulates both matrix stiffness and the cellular responses to stiffness.
CD98hc is highly expressed in many tumors of different origins as well as in established tumor cell lines. Our results showing that loss of CD98hc protects against tumor formation is in good agreement with recent studies on more invasive and non-accessible tumors from the intestinal epithelium (15,29). Indeed, Nguyen et al. showed that CD98hc role in the gut tumorigenesis is partly due to an effect on epithelial intrinsic cell proliferation. We previously showed that the integrin-interacting function of CD98hc can mediate cellular proliferation in teratocarcinomas and lymphocytes (4,30). Notably, we report that, conversely to the gut, CD98hc in the skin does not perturb TPA-induced immune responses, involved during tumorigenesis.
Furthermore, we show, for the first time, that not only CD98hc acts on tumor progression, but that its loss causes tumor regression. Importantly, CD98hc also forms the heavy chain of an amino acid co-transporter that can support the nutrient requirements of tumors (31) and consequent mTOR activation (17). Thus, it is likely that the capacity of CD98hc to support metabolic re-programming combined with its capacity to amplify mechanotransduction contribute to its critical role both in the clonal expansion of lymphocytes (30,32) and in tumorigenesis.
The tumor microenvironment is a mechanically tunable meshwork comprising extracellular matrix (ECM) proteins, a heterogeneous population of stromal cells and secreted soluble factors. The modifications of the mechanical properties of this microenvironment, which are monitored by ECM receptors such as integrins, play an important role during tumor progression (33). Here, we provide a novel function of CD98hc independent of its required role in immune response linked to epithelial tumorigenesis. Our data are in good agreement with studies on breast carcinoma, showing the critical role of integrin-signaling in mechanosensing during tumorigenesis. CD98hc function as a co-receptor of integrins probably participates to integrin-signaling in mechanosensing in many cancer types.
SCC is the second most common skin cancer (1) and its pathogenesis is tied to integrin expression (34),35). Elegant studies point to an important role for keratinocyte α5β1 integrin rather than α3β1 or α2β1 in SCCs formation (36). Most importantly, these studies show that the tumors arise from the underlying basal-layer cells (37). Newer studies point to dysfunction in environmental sensing, an integrin-dependent process, as a contributor to tumor formation and progression (19,38,39) . We now find that CD98hc loss drastically compromises environment stiffness sensing and conversely leads to a marked reduction in matrix stiffness.
Importantly, the physiological elasticity of skin has been long evaluated, using other types of technologies, to range between 5 and 10 kPa (20,21,22), gel compliance we used in our in vitro studies. In the AFM settings we utilized, this value reaches 0.53 MPa on average. This apparent discrepancy can be explained by recent work from Akhtar et al. (40) showing that elastic modulus of the constituent ECM molecules is higher than the one of the connective tissues themselves. The dermis is of fibrillar nature, and the nanoscopic AFM probe we used in vivo is likely to find very distinct areas, such as fibers, upon which to indent. This leads to measurement of elastic modulus reaching values in the MPa range. Finally, in good agreement with the explanation above, our data suggest that, in the absence of CD98hc, the ECM components responsible for structural rigidity, such as collagen content, might be modulated, leading to an overall decrease in stiffness and altering the skin mechanical properties
Integrin-mediated adhesions are intrinsically mechanosensitive and a large body of data implicates integrins in sensing mechanical forces (39,41). Piccolo’s laboratory pioneered the notion that mechanical signals and cell shape are converted into biochemical responses by two related transcription factors, YAP and TAZ (26,42). In particular, YAP and TAZ are nuclear relays of mechanical signals exerted by ECM rigidity (25) and mCTGF, a well characterized YAP/TAZ target, induces collagen deposition. CD98hc loss reduces ROCK activity in vivo resulting in decreased signaling of YAP/TAZ, and concomitantly a decrease of mCTGF expression, suggesting a role for CD98hc in collagen deposition. Importantly, cells deficient for CD98hc lose their capacity to ‘read’ physical and mechanical cues. The absence of CD98hc makes cells on a stiff ECM behave as if they were on a soft one. Thus, CD98hc tunes cellular stiffness sensing during tumorigenesis via the integrin/ROCK/YAP/TAZ pathway.
In summary, our data establish a central role for CD98hc in chemically-induced tumor development and progression in skin and show that removal of CD98hc can induce tumor regression. We demonstrate that CD98hc mediates this process by acting as an amplifier of integrin-mediated mechanotransduction and that the gain in stiffness sensing provided by CD98hc forms the core of a positive feedback loop that increases the stiffness of the microenvironment and the cellular responses. Lastly, we implicate YAP/TAZ as a RhoA/ROCK regulated pathway that contributes to CD98hc-mediated tumorigenesis. These studies also highlight the potential synergic effect of blocking CD98hc, in an anti-cancer therapy, allowing targeting both tumor cell proliferation and tumor environment matrix stiffness.
Supplementary Material
Acknowledgments
We thank the IRCAN core facilities (microscopy and animal facility) for technical help.
This study was supported by grants from INSERM InCa/AVENIR (R08227AS), from Association pour la Recherche sur le Cancer (ARC R10159AA), and from Agence Nationale de la Recherche (ANR R09101AS), and through the “Investments for the Future” LABEX SIGNALIFE : program reference # ANR-11-LABX-0028-01. E. Boulter was the recipient of a postdoctoral fellowship from the Ligue Nationale Contre le Cancer (RAB 12007 ASA) and a Marie Curie IRG from the European Union FP7 (agreement 276945).
Footnotes
The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Xie J. Molecular Biology of Basal and Squamous Cell Carcinomas. In: Reichrath J, editor. Sunlight, Vitamin D and Skin Cancer [Internet] New York: Springer; 2008. [cited 2013 Apr 26]. pp. 241–251. Available from: http://link.springer.com/chapter/10.1007/978-0-387-77574-6_19. [Google Scholar]
- 2.Cantor JM, Ginsberg MH. CD98 at the Crossroads of Adaptive Immunity and Cancer. [cited 2012 Apr 19];J Cell Sci [Internet] 2012 doi: 10.1242/jcs.096040. Available from: http://jcs.biologists.org/content/early/2012/04/10/jcs.096040. [DOI] [PMC free article] [PubMed]
- 3.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 4.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci USA. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262:720–725. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- 6.Shishido T, Uno S, Kamohara M, Tsuneoka-Suzuki T, Hashimoto Y, Enomoto T, et al. Transformation of BALB3T3 cells caused by over-expression of rat CD98 heavy chain (HC) requires its association with light chain: mis-sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer. 2000;87:311–316. doi: 10.1002/1097-0215(20000801)87:3<311::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Féral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, et al. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rintoul RC, Buttery RC, Mackinnon AC, Wong WS, Mosher D, Haslett C, et al. Cross-Linking CD98 Promotes Integrin-like Signaling and Anchorage-independent Growth. Mol Biol Cell. 2002;13:2841–2852. doi: 10.1091/mbc.01-11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, et al. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J Biol Chem. 2004;279:54731–54741. doi: 10.1074/jbc.M408700200. [DOI] [PubMed] [Google Scholar]
- 10.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulter E, Estrach S, Errante A, Pons C, Cailleteau L, Tissot F, et al. CD98hc (SLC3A2) regulation of skin homeostasis wanes with age. [cited 2013 Jan 11];J Exp Med [Internet] 2013 doi: 10.1084/jem.20121651. Available from: http://jem.rupress.org/content/early/2012/12/24/jem.20121651. [DOI] [PMC free article] [PubMed]
- 12.Montanez E, Piwko-Czuchra A, Bauer M, Li S, Yurchenco P, Fässler R. Analysis of Integrin Functions in Peri-Implantation Embryos, Hematopoietic System, and Skin. In: Cheresh David A, editor. Methods in Enzymology [Internet] Academic Press; 2007. [cited 2013 Feb 4]. pp. 239–289. Available from: http://www.sciencedirect.com/science/article/pii/S0076687907260124. [DOI] [PubMed] [Google Scholar]
- 13.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, et al. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and β-Catenin Activation to Induce Epidermal Hyperplasia and Tumor Growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Seminars in Cancer Biology. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen HTT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devés R, Boyd CA. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- 17.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jachowicz J, McMullen R, Prettypaul D. Indentometric analysis of in vivo skin and comparison with artificial skin models. Skin Res Technol. 2007;13:299–309. doi: 10.1111/j.1600-0846.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy Proteins on Stretchy Substrates: The Important Elements of Integrin-Mediated Rigidity Sensing. Developmental Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crichton ML, Donose BC, Chen X, Raphael AP, Huang H, Kendall MAF. The viscoelastic, hyperelastic and scale dependent behaviour of freshly excised individual skin layers. Biomaterials. 2011;32:4670–4681. doi: 10.1016/j.biomaterials.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 24.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MA. Integrins and Extracellular Matrix in Mechanotransduction. [cited 2013 Jan 23];Cold Spring Harb Perspect Biol [Internet] 2010 :2. doi: 10.1101/cshperspect.a005066. Available from: http://cshperspectives.cshlp.org/content/2/12/a005066. [DOI] [PMC free article] [PubMed]
- 27.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J Cell Sci. 2012;125:5974–5983. doi: 10.1242/jcs.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HTT, Dalmasso G, Yan Y, Laroui H, Charania M, Ingersoll S, et al. Intestinal epithelial cell-specific CD98 expression regulates tumorigenesis in Apc(Min/+) mice. Lab Invest. 2012;92:1203–1212. doi: 10.1038/labinvest.2012.83. [DOI] [PubMed] [Google Scholar]
- 30.Cantor J, Browne CD, Ruppert R, Féral CC, Fässler R, Rickert RC, et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat Immunol. 2009;10:412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCracken AN, Edinger AL. Nutrient transporters: the Achilles’ heel of anabolism. Trends in Endocrinology & Metabolism. 2013;24:200–208. doi: 10.1016/j.tem.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantor J, Slepak M, Ege N, Chang JT, Ginsberg MH. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J Immunol. 2011;187:851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 35.Owens DM, Broad S, Yan X, Benitah SA, Watt FM. Suprabasal α5β1 integrin expression stimulates formation of epidermal squamous cell carcinomas without disrupting TGFβ signaling or inducing spindle cell tumors. Molecular Carcinogenesis. 2005;44:60–66. doi: 10.1002/mc.20118. [DOI] [PubMed] [Google Scholar]
- 36.Owens DM, Watt FM. Influence of β1 Integrins on Epidermal Squamous Cell Carcinoma Formation in a Transgenic Mouse Model α3β1, but not α2β1, Suppresses Malignant Conversion. Cancer Res. 2001;61:5248–5254. [PubMed] [Google Scholar]
- 37.Owens DM, Watt FM. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer. 2003;3:444–451. doi: 10.1038/nrc1096. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 40.Akhtar R, Schwarzer N, Sherratt MJ, Watson REB, Graham HK, Trafford AW, et al. Nanoindentation of histological specimens: Mapping the elastic properties of soft tissues. J Mater Res. 2009;24:638–646. doi: 10.1557/JMR.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in Mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.