Abstract
Objectives:
We report on a chronic hemiparetic patient whose gait recovery was delayed until healing of an injured corticoreticulospinal tract (CRT), which was demonstrated on diffusion tensor tractography (DTT).
Case presentation:
A 71-year-old female presented with complete paralysis of the right extremities resulting from a spontaneous intracerebral hemorrhage. At 5 months after onset, when she was admitted for rehabilitation after undergoing rehabilitation at the previous university hospital, she presented with severe weakness of the right leg (manual muscle test: 0 ∼ 2- score) and could not even stand. She received comprehensive rehabilitative therapy for 32 months after the onset. Motor weakness of her right leg improved to the point that she was able to extend her knee on gravity-eliminated position at 11 months and against some resistance at 30 months after onset. She was able to walk independently at 30 months after onset.
Results:
The left CRT was discontinuous at the basal ganglia level on 5-month DTT. This discontinuation elongated to the cerebral cortex on 32-month DTT, whereas on 32-month DTT, the right CRT had become thicker compared with that on 5-month DTT.
Conclusions:
An injured CRT healed in a patient who was able to walk independently after approximately 2 years of rehabilitation starting 5 months after the onset of intracerebral hemorrhage.
Keywords: corticoreticulospinal tract, diffusion tensor tractography, gait, stroke
1. Introduction
Gait dysfunction, a common sequela of brain injury, is usually caused by problems such as motor weakness, somatosensory problem, and movement in coordination.[1–3] Approximately 20% to 30% of stroke patients do not regain gait ability; therefore, gait disability is a serious disabling sequelae of stroke.[4,5] Most gait recovery, and also motor recovery after stroke, occurs within 3 months after onset.[4,6] Stroke patients can walk when the motor function recovers in the hip and knee joint of the leg at least to the degree of being able to lift against gravity. Several studies have reported gait recovery by the recovery of injured corticospinal tract (CST) or corticoreticulospinal tract (CRT).[7–9] Jang et al[7] found thickening of CRT in the unaffected side showed association with gait function in 54 chronic stroke patients. In 2016, Jang and Kwon[9] reported that thickening of injured CST (perilesional recovery) at the early-stage rehabilitation contributed to gait function in a patient with a pontine infarct. This suggests that detailed knowledge about gait recovery could aid more stroke patients in regaining their gait ability even for stroke patients who could not walk after 3 months from onset.[10,11]
The CRT originates from the premotor cortex descended reticular formation in the brainstem. It contributes to gait function by controlling the proximal muscles of the extremities and axial muscles.[12] Therefore, it is important to study the CRT in patients with gait disturbance. Recent diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has a unique advantage in evaluation of microstructural integrity of white matter by detection of water diffusion properties.[13] A technique for identifying the CRT using selection of fibers passing through regions of interest (ROIs) was reported in 2012.[14] After introduction of identification of the CRT by DTT, a few studies have reported on the recovery of an injured CRT with gait function in stroke patients.[8,15,16] However, it has not been clearly elucidated so far.
In the current study, we report on a chronic hemiparetic patient whose gait recovery was delayed until healing of an injured CRT, which was demonstrated on DTT.
2. Case report
A 71-year-old right-handed female presented with complete paralysis of the right extremities [Medical Research Council (MRC): 0/5] at the onset of a spontaneous intracerebral hemorrhage (ICH) (Table 1).[17] The volume of hemorrhage (22950.7 mm3), was measured by Insight Toolkit - SNake Automatic Partitioning program (University of Pennsylvania, Philadelphia, PA). No previous medical history of neurological, physical, or psychiatric illness was observed except for hypertension and diabetes. Five months after onset, she was admitted to the rehabilitation department of a university hospital after undergoing rehabilitation at a different university hospital. Brain magnetic resonance (MR) images taken upon admission showed a leukomalactic lesion in the left corona radiata and basal ganglia (Fig. 1A). For measurement of gait function, functional ambulation category (FAC, full mark: 5 points) was measured. She presented with mild conduction aphasia, severe weakness of the right leg (MRC: hip flexor; 2-, knee extensor: 0, and ankle dorsiflexor: 0) and could not even stand (FAC: 0 point) (Table 1). She began comprehensive rehabilitative therapy, which included neurotropic drugs (methylphenidate, pramipexole, amantadine, levodopa, and venlafaxine), movement therapy, and neuromuscular electrical stimulation of the right knee extensor and ankle dorsiflexor.[15,18–22] Movement therapy was conducted 30 min/d and 5 times per week in our physical and occupational therapy department. Her rehabilitation was continued until 32 months after onset at the inpatient clinic of our hospital and 2 other local rehabilitation hospitals, and the outpatient clinic of the rehabilitation department of our hospital. Motor weakness of her right leg improved to the point that she was able to extend her knee on gravity-eliminated position at 11 months and against some resistance at 30 months after onset. She was able to walk independently at 30 months after her stroke (FAC: 3.5 points). Seven age and sex-matched normal control subjects (mean age: 67.9 ± 5.0 years, range: 62–77 years) with no history of neurological disease were recruited for this study. The patient and all normal control subjects provided informed consent, and the study protocol was approved by Yeungnam University Hospital institutional review board.
Table 1.
Changes in motor function and diffusion tensor tractography parameters of the patient.
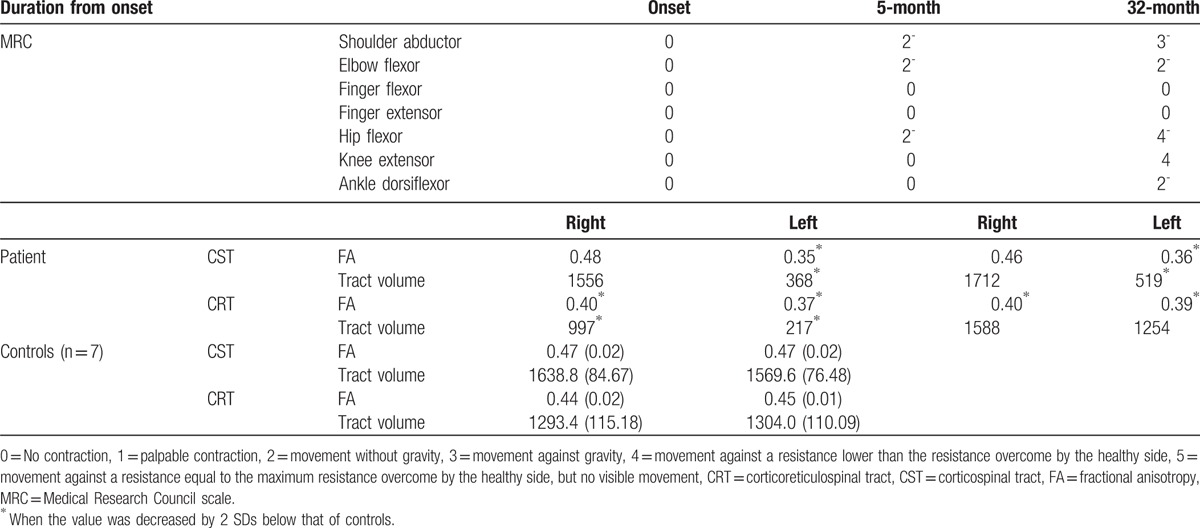
Figure 1.
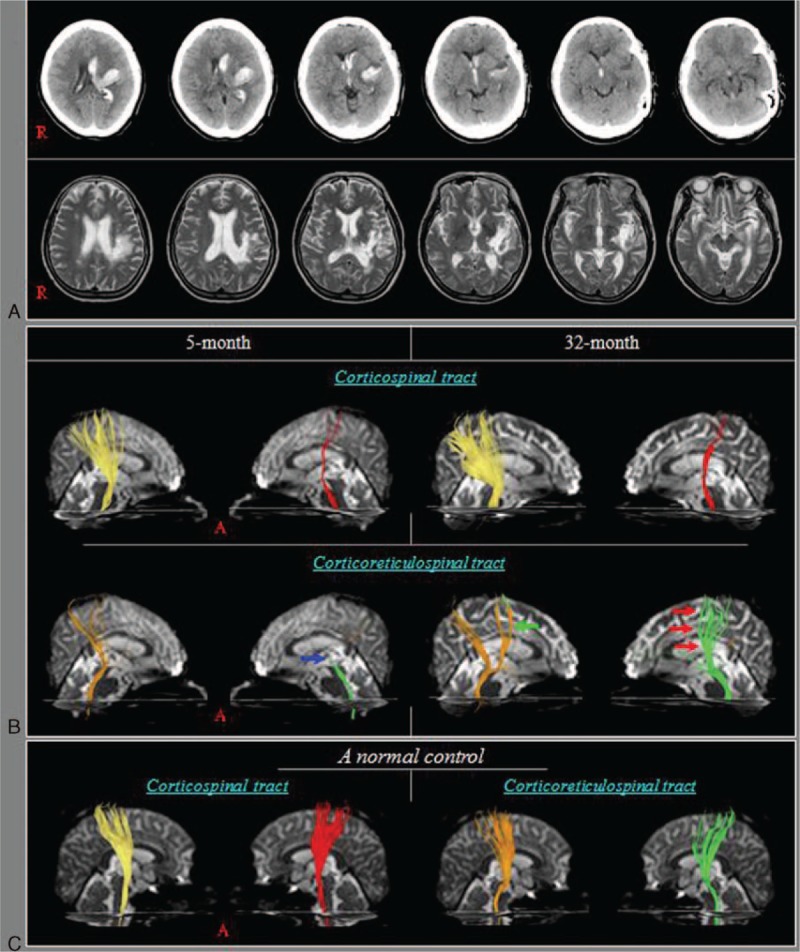
A, Brain computed tomography (CT) images show hematomas in the left corona radiata and basal ganglia at onset and T2-weighted magnetic resonance (MR) images show a leukomalactic lesion in the left corona radiata and basal ganglia at 5 months after onset. B, Results of diffusion tensor tractography (DTT). The left corticospinal tract (CST) shows narrowing compared with the right CST on 5-month DTT. Similar findings were observed for both CSTs on 32-month DTT. The left corticoreticulospinal tract (CRT) shows discontinuation at the basal ganglia level on 5-month DTT (blue arrow) and this discontinuation is elongated to the cerebral cortex on 32-month DTT (red arrows), whereas on 32-month DTT, the right CRT has become thicker than it was on the 5-month DTT (green arrow). C, Results of DTT for the CST and CRT in a normal control.
2.1. Diffusion tensor tractography
Diffusion tensor imaging scanning was performed at 5 and 32 months after onset, using a 6-channel head coil on a 1.5-T Philips Gyroscan Intera (Philips, Best, the Netherlands) with 32 gradients. Seventy contiguous slices and imaging parameters were acquired as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192, field of view = 240 × 240 mm2, TR = 10,398 milliseconds, TE = 72 milliseconds, EPI factor = 59 and b = 1000 s/mm2, NEX = 1, and a slice thickness of 2.5 mm. Fiber tracking was performed using the fiber assignment continuous tracking algorithm implemented within the DTI task card software (Philips Extended MR Work Space 2.6.3) (threshold fractional anisotropy = 0.15, angle = 27o). Each DTI replication was intraregistered to the baseline “b0” images for correction of residual Eddy-current image distortions and head motion effect, using a diffusion registration package (Philips Medical Systems). For reconstruction of the CST, 2 ROIs were placed on the upper (ROI 1) and lower pons (ROI 2) on the axial image (portion of the anterior blue color). For reconstruction of the CRT, reticular formation of the medulla (ROI 1), and tegmentum of the midbrain (ROI 2) on the axial image were used. Fractional anisotropy (FA) and tract volume of the CST and CRT were measured. DTT parameter values varying more than 2 SDs from normal control values were defined as significant differences.
On 5-month DTT, the entire right CST pathway was intact. In contrast, the left CST was narrowed compared with the right CST, and similar findings were observed for both CSTs on 32-month DTT compared with 5-month DTT. Regarding the CRT, the left CRT was discontinuous at the basal ganglia level on 5-month DTT, and this discontinuation elongated to the cerebral cortex on 32-month DTT. In addition, the right CRT presented integrity between the cortex and brainstem on 5-month DTT, and on 32-month DTT, the right DTT had become thicker compared with the 5-month DTT. Regarding DTT parameters, results of the DTT parameters are summarized in Table 1. The FA values of the CST and CRT were similar in both hemispheres on both 5 and 32-month DTTs. However, the tract volumes were increased in the CST and CRT in both hemispheres and the order of the increment of tract volume was as follows: the left CRT: 217→1254; the right CRT: 997→1588; the left CST: 368→519; and the right CST: 1556→1712. Compared with normal controls, significant differences in FA, and tract volume of the left CST on both 5 and 32-month DTT were observed. Regarding the CRT, significant differences in FA and tract volume of both CRTs on 5-month DTT were observed. In addition, on 32-month DTT, both CRTs in FA were lower than that of normal controls, but the tract volume of both CRTs did not differ significantly from the normal controls.
3. Discussion
In this study, we investigated the changes of the CST and CRT in a patient who was able to walk independently after approximately 2 years of rehabilitation starting 5 months after a cerebral hemorrhage. The results of DTT parameters appeared to be consistent with the changes of DTT configurations: the discontinuous left CRT elongated to the cerebral cortex and the right CRT became thicker on 32-month DTT. Because neither CST improved, we believe that the recovery of the injured left CRT was most attributed to regaining gait ability in this patient. The right CRT also appeared to contribute to regaining gait ability, apparently consistent with a previous study showing that activation of the unaffected CRT could contribute to gait recovery in stroke patients with severe injuries of the affected CST and CRT.[7] To the best of our knowledge, this is the first study to suggest recovery of the injured CRT contributes to regaining gait ability in chronic hemiparetic patients.
Since the introduction of DTT for the CRT, a few studies have reported on the recovery of an injured CRT.[8,15,16] In 2013, Yeo and Jang[8] reported on a patient who showed recovery of a discontinued CRT in the affected hemisphere during 3 weeks from 3 weeks after the onset of ICH. Subsequently, in 2014, a patient whose injured CRT had recovered by transcallosal fibers during 10 weeks from 6 weeks after the onset of ICH was reported.[15] In 2015, Jang et al[16] reported on a patient who developed reorganization of an injured CRT to the medial area over a 4-week period starting 6 weeks after a cerebral infarct. By contrast, we demonstrated the recovery of an injured CRT in a chronic patient who was able to walk independently after approximately 2 years of rehabilitation beginning 5 months after the ICH.
Several limitations of this study should be considered. First, because this is a single-case study, we could not study differences in predictors of gait recovery such as age, type of stroke, or type of therapy. Second, results of DTT might present false-positive and negative results due to the hemorrhage or a malatic cavity.[23] Third, although gait is involved in many neural tracts including extrapyramidal motor tracts, such as the rubrospinal tract and the vestibulospinal tract, we investigated only the CST and CRT. Therefore, further studies including larger case numbers and for overcoming the limitations should be warranted.
In conclusion, recovery of an injured CRT was found in a patient who was able to walk independently after approximately 2 years of rehabilitation starting 5 months after the ICH.
Footnotes
Abbreviations: CRT = corticoreticulospinal tract, CST = corticospinal tract, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, FA = fractional anisotropy, ICH = intracerebral hemorrhage, MRC = Medical Research Council, ROI = region of interest.
Funding: This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A2A01004073).
The authors have no conflicts of interest to disclose.
References
- 1.Keenan MA, Perry J, Jordan C. Factors affecting balance and ambulation following stroke. Clin Orthop Relat Res 1984; 165–171. [PubMed] [Google Scholar]
- 2.Friedman PJ. Gait recovery after hemiplegic stroke. Int Disabil Stud 1990; 12:119–122. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen HS, Nakayama H, Raaschou HO, et al. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1995; 76:27–32. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1995; 76:406–412. [DOI] [PubMed] [Google Scholar]
- 5.Kim CH. Motor recovery after stroke. J Korean Acad Rehab 1995; 19:55–61. [Google Scholar]
- 6.Wade DT, Wood VA, Hewer RL. Recovery after stroke: the first 3 months. J Neurol Neurosurg Psychiatry 1985; 48:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SH, Chang CH, Lee J, et al. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 2013; 44:1099–1104. [DOI] [PubMed] [Google Scholar]
- 8.Yeo SS, Jang SH. Recovery of an injured corticospinal tract and an injured corticoreticular pathway in a patient with intracerebral hemorrhage. NeuroRehabilitation 2013; 32:305–309. [DOI] [PubMed] [Google Scholar]
- 9.Jang SH, Kwon HG. Recovery of an injured corticospinal tract during the early stage of rehabilitation following pontine infarction. Neural Regen Res 2016; 11:519–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon H, Jang SH. Delayed recovery of gait function in a patient with intracerebral haemorrhage. J Rehabil Med 2012; 44:378–380. [DOI] [PubMed] [Google Scholar]
- 11.Seo JP, Lee MY, Kwon YH, et al. Delayed gait recovery in a stroke patient. Neural Regen Res 2013; 8:1514–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama K, Mori F, Nakajima K, et al. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res 2004; 143:239–249. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45:265–269. [DOI] [PubMed] [Google Scholar]
- 14.Yeo SS, Chang MC, Kwon YH, et al. Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett 2012; 508:9–12. [DOI] [PubMed] [Google Scholar]
- 15.Jang SH, Yeo SS. Recovery of an injured corticoreticular pathway via transcallosal fibers in a patient with intracerebral hemorrhage. BMC Neurol 2014; 14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SH, Lee J, Lee HD. Peri-infarct reorganization of an injured corticoreticulospinal tract in a patient with cerebral infarct. Int J Stroke 2015; 10:E62–E63. [DOI] [PubMed] [Google Scholar]
- 17.Council MR. Aids to the Examination of the Peripheral Nervous System: Her Majesty's Stationery Office, 1976. [Google Scholar]
- 18.Scheidtmann K, Fries W, Muller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358:787–790. [DOI] [PubMed] [Google Scholar]
- 19.Hesse S, Werner C. Poststroke motor dysfunction and spasticity: novel pharmacological and physical treatment strategies. CNS Drugs 2003; 17:1093–1107. [DOI] [PubMed] [Google Scholar]
- 20.Rosser N, Floel A. Pharmacological enhancement of motor recovery in subacute and chronic stroke. NeuroRehabilitation 2008; 23:95–103. [PubMed] [Google Scholar]
- 21.Molina-Luna K, Pekanovic A, Rohrich S, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 2009; 4:e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang MC, Jung YJ, Jang SH. Motor recovery via transcallosal and transpontine fibers in a patient with intracerebral hemorrhage. Am J Phys Med Rehabil 2014; 93:708–713. [DOI] [PubMed] [Google Scholar]
- 23.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 2005; 360:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]


